Failure of the adaptive unfolded protein response in islets of obese mice is linked with abnormalities in β-cell gene expression and progression to diabetes
- PMID: 23274897
- PMCID: PMC3636637
- DOI: 10.2337/db12-0701
Failure of the adaptive unfolded protein response in islets of obese mice is linked with abnormalities in β-cell gene expression and progression to diabetes
Abstract
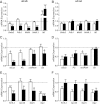
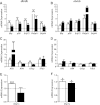
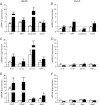
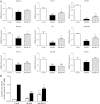
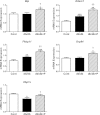
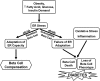
The normal β-cell response to obesity-associated insulin resistance is hypersecretion of insulin. Type 2 diabetes develops in subjects with β-cells that are susceptible to failure. Here, we investigated the time-dependent gene expression changes in islets of diabetes-prone db/db and diabetes-resistant ob/ob mice. The expressions of adaptive unfolded protein response (UPR) genes were progressively induced in islets of ob/ob mice, whereas they declined in diabetic db/db mice. Genes important for β-cell function and maintenance of the islet phenotype were reduced with time in db/db mice, whereas they were preserved in ob/ob mice. Inflammation and antioxidant genes displayed time-dependent upregulation in db/db islets but were unchanged in ob/ob islets. Treatment of db/db mouse islets with the chemical chaperone 4-phenylbutyric acid partially restored the changes in several β-cell function genes and transcription factors but did not affect inflammation or antioxidant gene expression. These data suggest that the maintenance (or suppression) of the adaptive UPR is associated with β-cell compensation (or failure) in obese mice. Inflammation, oxidative stress, and a progressive loss of β-cell differentiation accompany diabetes progression. The ability to maintain the adaptive UPR in islets may protect against the gene expression changes that underlie diabetes development in obese mice.
Figures


References
-
- Weir GC, Marselli L, Marchetti P, Katsuta H, Jung MH, Bonner-Weir S. Towards better understanding of the contributions of overwork and glucotoxicity to the beta-cell inadequacy of type 2 diabetes. Diabetes Obes Metab 2009;11(Suppl 4):82–90 - PubMed
-
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 - PubMed
-
- Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab 2008;10(Suppl 4):32–42 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

