Extending prednisolone treatment does not reduce relapses in childhood nephrotic syndrome
- PMID: 23274956
- PMCID: PMC3537218
- DOI: 10.1681/ASN.2012070646
Extending prednisolone treatment does not reduce relapses in childhood nephrotic syndrome
Abstract
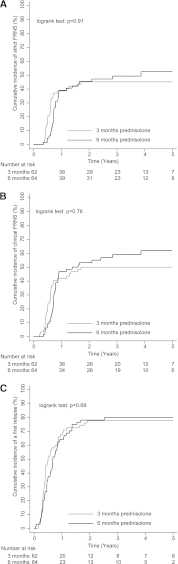
Prolonged prednisolone treatment for the initial episode of childhood nephrotic syndrome may reduce relapse rate, but whether this results from the increased duration of treatment or a higher cumulative dose remains unclear. We conducted a randomized, double-blind, placebo-controlled trial in 69 hospitals in The Netherlands. We randomly assigned 150 children (9 months to 17 years) presenting with nephrotic syndrome to either 3 months of prednisolone followed by 3 months of placebo (n=74) or 6 months of prednisolone (n=76), and median follow-up was 47 months. Both groups received equal cumulative doses of prednisolone (approximately 3360 mg/m(2)). Among the 126 children who started trial medication, relapses occurred in 48 (77%) of 62 patients who received 3 months of prednisolone and 51 (80%) of 64 patients who received 6 months of prednisolone. Frequent relapses, according to international criteria, occurred with similar frequency between groups as well (45% versus 50%). In addition, there were no statistically significant differences between groups with respect to the eventual initiation of prednisolone maintenance and/or other immunosuppressive therapy (50% versus 59%), steroid dependence, or adverse effects. In conclusion, in this trial, extending initial prednisolone treatment from 3 to 6 months without increasing cumulative dose did not benefit clinical outcome in children with nephrotic syndrome. Previous findings indicating that prolonged treatment regimens reduce relapses most likely resulted from increased cumulative dose rather than the treatment duration.
Figures



Comment in
-
Corticosteroid therapy for steroid-sensitive nephrotic syndrome in children: dose or duration?J Am Soc Nephrol. 2013 Jan;24(1):7-9. doi: 10.1681/ASN.2012111093. Epub 2012 Dec 14. J Am Soc Nephrol. 2013. PMID: 23243214 No abstract available.
References
-
- Eddy AA, Symons JM: Nephrotic syndrome in childhood. Lancet 362: 629–639, 2003 - PubMed
-
- Trompeter RS, Lloyd BW, Hicks J, White RH, Cameron JS: Long-term outcome for children with minimal-change nephrotic syndrome. Lancet 1: 368–370, 1985 - PubMed
-
- Bruneau S, Dantal J: New insights into the pathophysiology of idiopathic nephrotic syndrome. Clin Immunol 133: 13–21, 2009 - PubMed
-
- International Study of Kidney Disease in Children : The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International Study of Kidney Disease in Children. J Pediatr 98: 561–564, 1981 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

