Simvastatin protects hepatocytes from apoptosis by suppressing the TNF-α/caspase-3 signaling pathway in mice with burn injury
- PMID: 23275311
- PMCID: PMC4102342
- DOI: 10.1097/SLA.0b013e318273fdca
Simvastatin protects hepatocytes from apoptosis by suppressing the TNF-α/caspase-3 signaling pathway in mice with burn injury
Abstract
Objective: To investigate the liver cellular apoptosis in response to burn injury and find out if statin treatment can ameliorate this process. The hypothesis is that statin may modulate apoptosis-related gene expression and thereby reduce hepatocytic apoptosis after burn injury.
Methods: Mice were subjected to 30% full-thickness burn injury and then treated either with or without simvastatin. The livers were harvested for histological assessment and determinations of gene expression. To investigate the mechanism involved, tumor necrosis factor (TNF)-α and caspase-3 knockout (KO) mice were also used to evaluate the effects of burn injury and simvastatin treatment on burn-induced liver injury. The effects of simvastatin on TNF-α and caspase-3 expressions were also evaluated in cultured mouse hepatocytes.
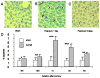
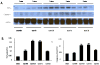
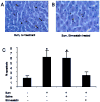
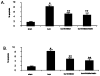
Results: Burn injury induced significant liver damage, which was indicated by striking levels of apoptosis. Simvastatin reduced the apoptotic index in the livers of mice with burn injury and this effect could be abrogated by TNF-α or caspase-3 inhibitors. Simvastatin also decreased burn-induced TNF-α and caspase-3 expression in the liver. TNF-α and caspase-3 KO mice demonstrated lower levels of apoptotic hepatocytes in response to burn, and simvastatin did not further decrease hepatocyte apoptosis in either strain of KO mice. An in vitro study demonstrated that simvastatin suppresses TNF-α and caspase-3 expression in primary cultures of mouse hepatocytes.
Conclusions: Simvastatin reduces mouse hepatocyte apoptosis by suppressing expression of the TNF-α/caspase-3 pathway.
Conflict of interest statement
None.
Figures


References
-
- Jeschke MG, Herndon DN, Ebener C, et al. Nutritional intervention high in vitamins, protein, amino acids, and omega3 fatty acids improves protein metabolism during the hypermetabolic state after thermal injury. Arch Surg. 2001;136(11):1301–6. - PubMed
-
- Sener G, Kabasakal L, Cetinel S, et al. Leukotriene receptor blocker montelukast protects against burn-induced oxidative injury of the skin and remote organs. Burns. 2005;31(5):587–96. - PubMed
-
- Zhou ZB, Ding HQ, Qin FJ, et al. Effect of Zn7-metallothionein on oxidative stress in liver of rats with severe thermal injury. Acta Pharmacol Sin. 2003;24(8):764–70. - PubMed
-
- Sener G, Sehirli AO, Gedik N, et al. Rosiglitazone, a PPAR-gamma ligand, protects against burn-induced oxidative injury of remote organs. Burns. 2007;33(5):587–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

