Deletion of leucine zipper tumor suppressor 2 (Lzts2) increases susceptibility to tumor development
- PMID: 23275340
- PMCID: PMC3567628
- DOI: 10.1074/jbc.M112.417568
Deletion of leucine zipper tumor suppressor 2 (Lzts2) increases susceptibility to tumor development
Abstract
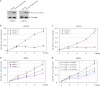
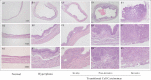
Using an Lzts2 knock-out mouse model, we characterized the biological role of Lzts2 in tumorigenesis. Both heterozygous and homozygous deletion of the Lzts2-targeted allele in mice shows an increased incidence in spontaneous tumor development, although Lzts2 homozygous knock-out mice show significantly higher incidences than heterozygous mice. Treatment of Lzts2-deficient mice with a carcinogen, N-butyl-N-(4-hydroxybutyl) nitrosamine, increases the susceptibility to N-butyl-N-(4-hydroxybutyl) nitrosamine-induced bladder carcinoma development. Examination of human prostate cancer tissue specimens shows a reduction of LZTS2 protein expression in prostate cancer cells. Further analyses of mouse embryonic fibroblasts isolated from Lzts2 knock-out embryos show that loss of Lzts2 enhances cell growth. These data provide the first line of evidence demonstrating that deletion of Lzts2 increases susceptibility to spontaneous and carcinogen-induced tumor development.
Figures





Similar articles
-
LZTS2 and PTEN collaboratively regulate ß-catenin in prostatic tumorigenesis.PLoS One. 2017 Mar 21;12(3):e0174357. doi: 10.1371/journal.pone.0174357. eCollection 2017. PLoS One. 2017. PMID: 28323888 Free PMC article.
-
High susceptibility of p53(+/-) knockout mice in N-butyl-N-(4-hydroxybutyl)nitrosamine urinary bladder carcinogenesis and lack of frequent mutation in residual allele.Cancer Res. 1998 Sep 1;58(17):3806-11. Cancer Res. 1998. PMID: 9731488
-
Fez1/Lzts1-deficient mice are more susceptible to N-butyl-N-(4-hydroxybutil) nitrosamine (BBN) carcinogenesis.Carcinogenesis. 2008 Apr;29(4):846-8. doi: 10.1093/carcin/bgn006. Epub 2008 Jan 12. Carcinogenesis. 2008. PMID: 18192690
-
Review: BBN as an urothelial carcinogen.In Vivo. 2012 Jul-Aug;26(4):727-39. In Vivo. 2012. PMID: 22773588 Review.
-
The N-butyl-N-4-hydroxybutyl Nitrosamine Mouse Urinary Bladder Cancer Model.Methods Mol Biol. 2018;1655:155-167. doi: 10.1007/978-1-4939-7234-0_13. Methods Mol Biol. 2018. PMID: 28889385 Review.
Cited by
-
Crosstalking between androgen and PI3K/AKT signaling pathways in prostate cancer cells.J Biol Chem. 2015 Jan 30;290(5):2759-68. doi: 10.1074/jbc.M114.607846. Epub 2014 Dec 19. J Biol Chem. 2015. PMID: 25527506 Free PMC article.
-
LZTS2 and PTEN collaboratively regulate ß-catenin in prostatic tumorigenesis.PLoS One. 2017 Mar 21;12(3):e0174357. doi: 10.1371/journal.pone.0174357. eCollection 2017. PLoS One. 2017. PMID: 28323888 Free PMC article.
-
The role of SH3RF2 in lung squamous cell carcinoma and M2 polarization: insights into LZTS2 ubiquitination.Biol Direct. 2025 Jul 17;20(1):87. doi: 10.1186/s13062-025-00677-0. Biol Direct. 2025. PMID: 40676695 Free PMC article.
-
Long non-coding RNA linc00921 suppresses tumorigenesis and epithelial-to-mesenchymal transition of triple-negative breast cancer via targeting miR-9-5p/LZTS2 axis.Hum Cell. 2022 May;35(3):909-923. doi: 10.1007/s13577-022-00685-6. Epub 2022 Feb 18. Hum Cell. 2022. PMID: 35179718 Free PMC article.
-
Clinical landscape of TP73 structural variants in ATL patients.Leukemia. 2023 Dec;37(12):2502-2506. doi: 10.1038/s41375-023-02059-9. Epub 2023 Oct 20. Leukemia. 2023. PMID: 37864123 Free PMC article. No abstract available.
References
-
- Cabeza-Arvelaiz Y., Thompson T. C., Sepulveda J. L., Chinault A. C. (2001) LAPSER1. A novel candidate tumor suppressor gene from 10q24.3. Oncogene 20, 6707–6717 - PubMed
-
- Vecchione A., Ishii H., Shiao Y. H., Trapasso F., Rugge M., Tamburrino J. F., Murakumo Y., Alder H., Croce C. M., Baffa R. (2001) Fez1/Lzts1 alterations in gastric carcinoma. Clin. Cancer Res. 7, 1546–1552 - PubMed
-
- Vecchione A., Baldassarre G., Ishii H., Nicoloso M. S., Belletti B., Petrocca F., Zanesi N., Fong L. Y., Battista S., Guarnieri D., Baffa R., Alder H., Farber J. L., Donovan P. J., Croce C. M. (2007) Fez1/Lzts1 absence impairs Cdk1/Cdc25C interaction during mitosis and predisposes mice to cancer development. Cancer Cell 11, 275–289 - PMC - PubMed
-
- Li J., Yen C., Liaw D., Podsypanina K., Bose S., Wang S. I., Puc J., Miliaresis C., Rodgers L., McCombie R., Bigner S. H., Giovanella B. C., Ittmann M., Tycko B., Hibshoosh H., Wigler M. H., Parsons R. (1997) PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275, 1943–1947 - PubMed
-
- Rasheed B. K., McLendon R. E., Friedman H. S., Friedman A. H., Fuchs H. E., Bigner D. D., Bigner S. H. (1995) Chromosome 10 deletion mapping in human gliomas. A common deletion region in 10q25. Oncogene 10, 2243–2246 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

