NADPH:quinone oxidoreductase 1 regulates host susceptibility to ozone via isoprostane generation
- PMID: 23275341
- PMCID: PMC3576073
- DOI: 10.1074/jbc.M112.438440
NADPH:quinone oxidoreductase 1 regulates host susceptibility to ozone via isoprostane generation
Abstract
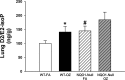
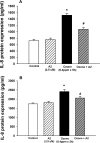
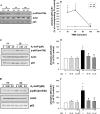
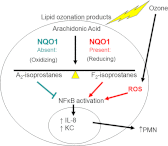
NADPH:quinone oxidoreductase 1 (NQO1) is recognized as a major susceptibility gene for ozone-induced pulmonary toxicity. In the absence of NQO1 as can occur by genetic mutation, the human airway is protected from harmful effects of ozone. We recently reported that NQO1-null mice are protected from airway hyperresponsiveness and pulmonary inflammation following ozone exposure. However, NQO1 regenerates intracellular antioxidants and therefore should protect the individual from oxidative stress. To explain this paradox, we tested whether in the absence of NQO1 ozone exposure results in increased generation of A(2)-isoprostane, a cyclopentenone isoprostane that blunts inflammation. Using GC-MS, we found that NQO1-null mice had greater lung tissue levels of D(2)- and E(2)-isoprostanes, the precursors of J(2)- and A(2)-isoprostanes, both at base line and following ozone exposure compared with congenic wild-type mice. We confirmed in primary cultures of normal human bronchial epithelial cells that A(2)-isoprostane inhibited ozone-induced NF-κB activation and IL-8 regulation. Furthermore, we determined that A(2)-isoprostane covalently modified the active Cys(179) domain in inhibitory κB kinase in the presence of ozone in vitro, thus establishing the biochemical basis for A(2)-isoprostane inhibition of NF-κB. Our results demonstrate that host factors may regulate pulmonary susceptibility to ozone by regulating the generation of A(2)-isoprostanes in the lung. These observations provide the biochemical basis for the epidemiologic observation that NQO1 regulates pulmonary susceptibility to ozone.
Figures



References
-
- (1996) Health effects of outdoor air pollution. Part 2. Committee of the Environmental and Occupational Health Assembly of the American Thoracic Society. Am. J. Respir. Crit. Care Med. 153, 477–498 - PubMed
-
- Peden D. B. (2005) The epidemiology and genetics of asthma risk associated with air pollution. J. Allergy Clin. Immunol. 115, 213–219 - PubMed
-
- Trasande L., Thurston G. D. (2005) The role of air pollution in asthma and other pediatric morbidities. J. Allergy Clin. Immunol. 115, 689–699 - PubMed
-
- Foster W. M., Brown R. H., Macri K., Mitchell C. S. (2000) Bronchial reactivity of healthy subjects: 18–20 h postexposure to ozone. J. Appl. Physiol. 89, 1804–1810 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

