Yes-associated protein isoform 1 (Yap1) promotes cardiomyocyte survival and growth to protect against myocardial ischemic injury
- PMID: 23275380
- PMCID: PMC3567650
- DOI: 10.1074/jbc.M112.436311
Yes-associated protein isoform 1 (Yap1) promotes cardiomyocyte survival and growth to protect against myocardial ischemic injury
Abstract
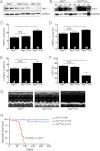
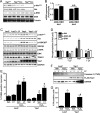
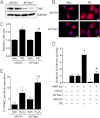
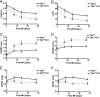
Yap1 is an important regulator of cardiomyocyte proliferation and embryonic heart development, yet the function of endogenous Yap1 in the adult heart remains unknown. We studied the role of Yap1 in maintaining basal cardiac function and in modulating injury after chronic myocardial infarction (MI). Cardiomyocyte-specific homozygous inactivation of Yap1 in the postnatal heart (Yap(F/F)Cre) elicited increased myocyte apoptosis and fibrosis, dilated cardiomyopathy, and premature death. Heterozygous deletion (Yap(+/F)Cre) did not cause an overt cardiac phenotype compared with Yap(F/F) control mice at base line. In response to stress (MI), nuclear Yap1 was found selectively in the border zone and not in the remote area of the heart. After chronic MI (28 days), Yap(+/F)Cre mice had significantly increased myocyte apoptosis and fibrosis, with attenuated compensatory cardiomyocyte hypertrophy, and further impaired function versus Yap(+/F) control mice. Studies in isolated cardiomyocytes demonstrated that Yap1 expression is sufficient to promote increased cell size and hypertrophic gene expression and protected cardiomyocytes against H(2)O(2)-induced cell death, whereas Yap1 depletion attenuated phenylephrine-induced hypertrophy and augmented apoptosis. Finally, we observed a significant decrease in cardiomyocyte proliferation in Yap(+/F)Cre hearts compared with Yap(+/F) controls after MI and demonstrated that Yap1 is sufficient to promote cardiomyocyte proliferation in isolated cardiomyocytes. Our findings suggest that Yap1 is critical for basal heart homeostasis and that Yap1 deficiency exacerbates injury in response to chronic MI.
Figures



Similar articles
-
miR-206 Mediates YAP-Induced Cardiac Hypertrophy and Survival.Circ Res. 2015 Oct 23;117(10):891-904. doi: 10.1161/CIRCRESAHA.115.306624. Epub 2015 Sep 2. Circ Res. 2015. PMID: 26333362 Free PMC article.
-
Yes-associated protein (YAP) mediates adaptive cardiac hypertrophy in response to pressure overload.J Biol Chem. 2019 Mar 8;294(10):3603-3617. doi: 10.1074/jbc.RA118.006123. Epub 2019 Jan 11. J Biol Chem. 2019. PMID: 30635403 Free PMC article.
-
Alpha-catenins control cardiomyocyte proliferation by regulating Yap activity.Circ Res. 2015 Jan 2;116(1):70-9. doi: 10.1161/CIRCRESAHA.116.304472. Epub 2014 Oct 10. Circ Res. 2015. PMID: 25305307 Free PMC article.
-
Regulation of Myocardial Cell Growth and Death by the Hippo Pathway.Circ J. 2016 Jun 24;80(7):1511-9. doi: 10.1253/circj.CJ-16-0476. Epub 2016 Jun 10. Circ J. 2016. PMID: 27302848 Review.
-
The Hippo pathway in the heart: pivotal roles in development, disease, and regeneration.Nat Rev Cardiol. 2018 Nov;15(11):672-684. doi: 10.1038/s41569-018-0063-3. Nat Rev Cardiol. 2018. PMID: 30111784 Review.
Cited by
-
Cardiac fibroblasts and mechanosensation in heart development, health and disease.Nat Rev Cardiol. 2023 May;20(5):309-324. doi: 10.1038/s41569-022-00799-2. Epub 2022 Nov 14. Nat Rev Cardiol. 2023. PMID: 36376437 Review.
-
Hippo/Yap Signaling in Cardiac Development and Regeneration.Curr Treat Options Cardiovasc Med. 2016 Jun;18(6):38. doi: 10.1007/s11936-016-0461-y. Curr Treat Options Cardiovasc Med. 2016. PMID: 27040401 Review.
-
The eIF2α serine 51 phosphorylation-ATF4 arm promotes HIPPO signaling and cell death under oxidative stress.Oncotarget. 2016 Aug 9;7(32):51044-51058. doi: 10.18632/oncotarget.10480. Oncotarget. 2016. PMID: 27409837 Free PMC article.
-
MST1 mediates doxorubicin-induced cardiomyopathy by SIRT3 downregulation.Cell Mol Life Sci. 2023 Aug 11;80(9):245. doi: 10.1007/s00018-023-04877-7. Cell Mol Life Sci. 2023. PMID: 37566283 Free PMC article.
-
Neddylation mediates ventricular chamber maturation through repression of Hippo signaling.Proc Natl Acad Sci U S A. 2018 Apr 24;115(17):E4101-E4110. doi: 10.1073/pnas.1719309115. Epub 2018 Apr 9. Proc Natl Acad Sci U S A. 2018. PMID: 29632206 Free PMC article.
References
-
- Narula J., Haider N., Virmani R., DiSalvo T. G., Kolodgie F. D., Hajjar R. J., Schmidt U., Semigran M. J., Dec G. W., Khaw B. A. (1996) Apoptosis in myocytes in end-stage heart failure. N. Engl. J. Med. 335, 1182–1189 - PubMed
-
- Olivetti G., Abbi R., Quaini F., Kajstura J., Cheng W., Nitahara J. A., Quaini E., Di Loreto C., Beltrami C. A., Krajewski S., Reed J. C., Anversa P. (1997) Apoptosis in the failing human heart. N. Engl. J. Med. 336, 1131–1141 - PubMed
-
- Mani K., Kitsis R. N. (2003) Myocyte apoptosis. Programming ventricular remodeling. J. Am. Coll. Cardiol. 41, 761–764 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL67724/HL/NHLBI NIH HHS/United States
- R01 HL091469/HL/NHLBI NIH HHS/United States
- P01 AG027211/AG/NIA NIH HHS/United States
- HL69020/HL/NHLBI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- HL91469/HL/NHLBI NIH HHS/United States
- P01 HL059139/HL/NHLBI NIH HHS/United States
- R01 HL112330/HL/NHLBI NIH HHS/United States
- AG27211/AG/NIA NIH HHS/United States
- R01 HL102738/HL/NHLBI NIH HHS/United States
- P01 HL069020/HL/NHLBI NIH HHS/United States
- HL59139/HL/NHLBI NIH HHS/United States
- R01 HL067724/HL/NHLBI NIH HHS/United States
- R21 AG042678/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

