DNA target sequence identification mechanism for dimer-active protein complexes
- PMID: 23275566
- PMCID: PMC3575837
- DOI: 10.1093/nar/gks1345
DNA target sequence identification mechanism for dimer-active protein complexes
Abstract
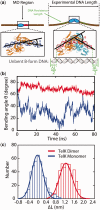
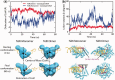
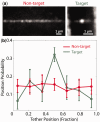
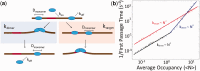
Sequence-specific DNA-binding proteins must quickly and reliably localize specific target sites on DNA. This search process has been well characterized for monomeric proteins, but it remains poorly understood for systems that require assembly into dimers or oligomers at the target site. We present a single-molecule study of the target-search mechanism of protelomerase TelK, a recombinase-like protein that is only active as a dimer. We show that TelK undergoes 1D diffusion on non-target DNA as a monomer, and it immobilizes upon dimerization even in the absence of a DNA target site. We further show that dimeric TelK condenses non-target DNA, forming a tightly bound nucleoprotein complex. Together with theoretical calculations and molecular dynamics simulations, we present a novel target-search model for TelK, which may be generalizable to other dimer and oligomer-active proteins.
Figures


Similar articles
-
An interlocked dimer of the protelomerase TelK distorts DNA structure for the formation of hairpin telomeres.Mol Cell. 2007 Sep 21;27(6):901-13. doi: 10.1016/j.molcel.2007.07.026. Mol Cell. 2007. PMID: 17889664 Free PMC article.
-
Modeling multi-component protein-DNA complexes: the role of bending and dimerization in the complex of p53 dimers with DNA.Protein Eng. 2001 Apr;14(4):233-43. doi: 10.1093/protein/14.4.233. Protein Eng. 2001. PMID: 11391015
-
Stability of the dimerization domain effects the cooperative DNA binding of short peptides.Biochemistry. 1999 Mar 30;38(13):4008-17. doi: 10.1021/bi9828829. Biochemistry. 1999. PMID: 10194313
-
Oligonucleotide binding proteins: the occurrence of dimer and multimer formation.Adv Exp Med Biol. 2012;747:91-104. doi: 10.1007/978-1-4614-3229-6_6. Adv Exp Med Biol. 2012. PMID: 22949113 Review.
-
Chemical approaches untangling sequence-specific DNA binding by proteins.Chemistry. 2002 Nov 15;8(22):5066-71. doi: 10.1002/1521-3765(20021115)8:22<5066::AID-CHEM5066>3.0.CO;2-0. Chemistry. 2002. PMID: 12412056 Review.
Cited by
-
DNA-Binding Activities of KSHV DNA Polymerase Processivity Factor (PF-8) Complexes.Viruses. 2025 Jan 29;17(2):190. doi: 10.3390/v17020190. Viruses. 2025. PMID: 40006945 Free PMC article.
-
High-Resolution "Fleezers": Dual-Trap Optical Tweezers Combined with Single-Molecule Fluorescence Detection.Methods Mol Biol. 2017;1486:183-256. doi: 10.1007/978-1-4939-6421-5_8. Methods Mol Biol. 2017. PMID: 27844430 Free PMC article.
-
Observing Protein One-Dimensional Sliding: Methodology and Biological Significance.Biomolecules. 2021 Nov 2;11(11):1618. doi: 10.3390/biom11111618. Biomolecules. 2021. PMID: 34827616 Free PMC article. Review.
-
Theory of Site-Specific DNA-Protein Interactions in the Presence of Nucleosome Roadblocks.Biophys J. 2018 Jun 5;114(11):2516-2529. doi: 10.1016/j.bpj.2018.04.039. Biophys J. 2018. PMID: 29874603 Free PMC article.

