Crystal structure of an intermediate of rotating dimers within the synaptic tetramer of the G-segment invertase
- PMID: 23275567
- PMCID: PMC3575834
- DOI: 10.1093/nar/gks1303
Crystal structure of an intermediate of rotating dimers within the synaptic tetramer of the G-segment invertase
Abstract
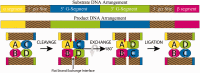
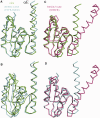
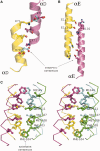
The serine family of site-specific DNA recombination enzymes accomplishes strand cleavage, exchange and religation using a synaptic protein tetramer. A double-strand break intermediate in which each protein subunit is covalently linked to the target DNA substrate ensures that the recombination event will not damage the DNA. The previous structure of a tetrameric synaptic complex of γδ resolvase linked to two cleaved DNA strands had suggested a rotational mechanism of recombination in which one dimer rotates 180° about the flat exchange interface for strand exchange. Here, we report the crystal structure of a synaptic tetramer of an unliganded activated mutant (M114V) of the G-segment invertase (Gin) in which one dimer half is rotated by 26° or 154° relative to the other dimer when compared with the dimers in the synaptic complex of γδ resolvase. Modeling shows that this rotational orientation of Gin is not compatible with its being able to bind uncleaved DNA, implying that this structure represents an intermediate in the process of strand exchange. Thus, our structure provides direct evidence for the proposed rotational mechanism of site-specific recombination.
Figures
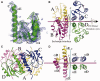

References
-
- Craig NL. Mobile DNA II. Washington, D.C: ASM Press; 2002.
-
- Guo F, Gopaul D, van Duyne G. Structure of Cre recombinase complexed with DNA in a site-specific recombination synapse. Nature. 1997;389:40–46. - PubMed
-
- Ghosh K, Lau C, Gupta K, Van Duyne G. Preferential synapsis of loxP sites drives ordered strand exchange in Cre-loxP site-specific recombination. Nat. Chem. Biol. 2005;1:275–282. - PubMed

