Epithelial transient receptor potential ankyrin 1 (TRPA1)-dependent adrenomedullin upregulates blood flow in rat small intestine
- PMID: 23275609
- PMCID: PMC3566615
- DOI: 10.1152/ajpgi.00356.2012
Epithelial transient receptor potential ankyrin 1 (TRPA1)-dependent adrenomedullin upregulates blood flow in rat small intestine
Abstract
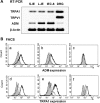
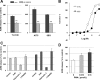
The functional roles of transient receptor potential (TRP) channels in the gastrointestinal tract have garnered considerable attention in recent years. We previously reported that daikenchuto (TU-100), a traditional Japanese herbal medicine, increased intestinal blood flow (IBF) via adrenomedullin (ADM) release from intestinal epithelial (IE) cells (Kono T et al. J Crohns Colitis 4: 161-170, 2010). TU-100 contains multiple TRP activators. In the present study, therefore, we examined the involvement of TRP channels in the ADM-mediated vasodilatatory effect of TU-100. Rats were treated intraduodenally with the TRP vanilloid type 1 (TRPV1) agonist capsaicin (CAP), the TRP ankyrin 1 (TRPA1) agonist allyl-isothiocyanate (AITC), or TU-100, and jejunum IBF was evaluated using laser-Doppler blood flowmetry. All three compounds resulted in vasodilatation, and the vasodilatory effect of TU-100 was abolished by a TRPA1 antagonist but not by a TRPV1 antagonist. Vasodilatation induced by AITC and TU-100 was abrogated by anti-ADM antibody treatment. RT-PCR and flow cytometry revealed that an IEC-6 cell line originated from the small intestine and purified IE cells expressed ADM and TRPA1 but not TRPV1. AITC increased ADM release in IEC cells remarkably, while CAP had no effect. TU-100 and its ingredient 6-shogaol (6SG) increased ADM release dose-dependently, and the effects were abrogated by a TRPA1 antagonist. 6SG showed similar TRPA1-dependent vasodilatation in vivo. These results indicate that TRPA1 in IE cells may play an important role in controlling bowel microcirculation via ADM release. Epithelial TRPA1 appears to be a promising target for the development of novel strategies for the treatment of various gastrointestinal disorders.
Figures





Similar articles
-
Daikenchuto (TU-100) ameliorates colon microvascular dysfunction via endogenous adrenomedullin in Crohn's disease rat model.J Gastroenterol. 2011 Oct;46(10):1187-96. doi: 10.1007/s00535-011-0438-2. Epub 2011 Aug 2. J Gastroenterol. 2011. PMID: 21808981
-
Allyl isothiocyanate, an activator of TRPA1, increases gastric mucosal blood flow through calcitonin gene-related peptide and adrenomedullin in anesthetized rats.J Pharmacol Sci. 2023 Apr;151(4):187-194. doi: 10.1016/j.jphs.2023.02.002. Epub 2023 Feb 21. J Pharmacol Sci. 2023. PMID: 36925217
-
Role of calcium ions in the positive interaction between TRPA1 and TRPV1 channels in bronchopulmonary sensory neurons.J Appl Physiol (1985). 2015 Jun 15;118(12):1533-43. doi: 10.1152/japplphysiol.00043.2015. Epub 2015 Apr 9. J Appl Physiol (1985). 2015. PMID: 25858491 Free PMC article.
-
Targeting TRPV1 and TRPA1: A feasible strategy for natural herbal medicines to combat postoperative ileus.Pharmacol Res. 2023 Oct;196:106923. doi: 10.1016/j.phrs.2023.106923. Epub 2023 Sep 13. Pharmacol Res. 2023. PMID: 37709183 Review.
-
Interactions between Chemesthesis and Taste: Role of TRPA1 and TRPV1.Int J Mol Sci. 2021 Mar 25;22(7):3360. doi: 10.3390/ijms22073360. Int J Mol Sci. 2021. PMID: 33806052 Free PMC article. Review.
Cited by
-
Efficacy and Safety of the Japanese Herbal Medicine Daikenchuto (DKT) in Elderly Fecal Incontinence Patients: A Prospective Study.J Anus Rectum Colon. 2022 Jan 28;6(1):32-39. doi: 10.23922/jarc.2021-038. eCollection 2022. J Anus Rectum Colon. 2022. PMID: 35128135 Free PMC article.
-
Enhanced anastomotic healing by Daikenchuto (TJ-100) in rats.Sci Rep. 2018 Jan 18;8(1):1091. doi: 10.1038/s41598-018-19550-4. Sci Rep. 2018. PMID: 29348453 Free PMC article.
-
Daikenchuto (TU-100) alters murine hepatic and intestinal drug metabolizing enzymes in an in vivo dietary model: effects of gender and withdrawal.Pharmacol Res Perspect. 2017 Oct;5(5):e00361. doi: 10.1002/prp2.361. Pharmacol Res Perspect. 2017. PMID: 28971602 Free PMC article.
-
Clinical pharmacology of daikenchuto assessed by transit analysis using radiopaque markers in patients with colon cancer undergoing open surgery: a multicenter double-blind randomized placebo-controlled study (JFMC39-0902 additional study).J Gastroenterol. 2016 Mar;51(3):222-9. doi: 10.1007/s00535-015-1100-1. Epub 2015 Jul 11. J Gastroenterol. 2016. PMID: 26162646 Clinical Trial.
-
Constipation and herbal medicine.Front Pharmacol. 2015 Apr 8;6:73. doi: 10.3389/fphar.2015.00073. eCollection 2015. Front Pharmacol. 2015. PMID: 25904866 Free PMC article.
References
-
- Akiho H, Nakamura K. Daikenchuto ameliorates muscle hypercontractility in a murine T-cell-mediated persistent gut motor dysfunction model. Digestion 83: 173– 179, 2011 - PubMed
-
- Arthur JS. MSK activation and physiological roles. Front Biosci 13: 5866– 5879, 2008 - PubMed
-
- Ashizuka S, Inagaki-Ohara K, Kuwasako K, Kato J, Inatsu H, Kitamura K. Adrenomedullin treatment reduces intestinal inflammation and maintains epithelial barrier function in mice administered dextran sulphate sodium. Microbiol Immunol 53: 573– 581, 2009 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

