Pharmacological inhibition of PAR2 with the pepducin P2pal-18S protects mice against acute experimental biliary pancreatitis
- PMID: 23275617
- PMCID: PMC3602677
- DOI: 10.1152/ajpgi.00296.2012
Pharmacological inhibition of PAR2 with the pepducin P2pal-18S protects mice against acute experimental biliary pancreatitis
Abstract
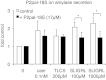
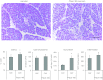
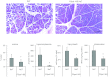
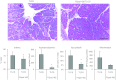
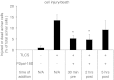
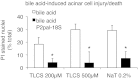
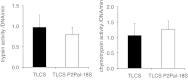
Pancreatic acinar cells express proteinase-activated receptor-2 (PAR2) that is activated by trypsin-like serine proteases and has been shown to exert model-specific effects on the severity of experimental pancreatitis, i.e., PAR2(-/-) mice are protected from experimental acute biliary pancreatitis but develop more severe secretagogue-induced pancreatitis. P2pal-18S is a novel pepducin lipopeptide that targets and inhibits PAR2. In studies monitoring PAR2-stimulated intracellular Ca(2+) concentration changes, we show that P2pal-18S is a full PAR2 inhibitor in acinar cells. Our in vivo studies show that P2pal-18S significantly reduces the severity of experimental biliary pancreatitis induced by retrograde intraductal bile acid infusion, which mimics injury induced by endoscopic retrograde cholangiopancreatography (ERCP). This reduction in pancreatitis severity is observed when the pepducin is given before or 2 h after bile acid infusion but not when it is given 5 h after bile acid infusion. Conversely, P2pal-18S increases the severity of secretagogue-induced pancreatitis. In vitro studies indicate that P2pal-18S protects acinar cells against bile acid-induced injury/death, but it does not alter bile acid-induced intracellular zymogen activation. These studies are the first to report the effects of an effective PAR2 pharmacological inhibitor on pancreatic acinar cells and on the severity of experimental pancreatitis. They raise the possibility that a pepducin such as P2pal-18S might prove useful in the clinical management of patients at risk for developing severe biliary pancreatitis such as occurs following ERCP.
Figures



References
-
- Adams MN, Ramachandran R, Yau MK, Suen JY, Fairlie DP, Hollenberg MD, Hooper JD. Structure, function and pathophysiology of protease activated receptors. Pharmacol Ther 130: 248–282, 2010 - PubMed
-
- Bohm SK, McConalogue K, Kong W, Bunnett NW. Proteinase-activated receptors: new functions for old enzymes. News Physiol Sci 13: 231–240, 1998 - PubMed
-
- Caruso R, Pallone F, Fina D, Gioia V, Peluso I, Caprioli F, Stolfi C, Perfetti A, Spagnoli LG, Palmieri G, Macdonald TT, Monteleone G. Protease-activated receptor-2 activation in gastric cancer cells promotes epidermal growth factor receptor trans-activation and proliferation. Am J Pathol 169: 268–278, 2006 - PMC - PubMed
-
- Fischer L, Gukovskaya AS, Young SH, Gukovsky I, Lugea A, Buechler P, Penninger JM, Friess H, Pandol SJ. Phosphatidylinositol 3-kinase regulates Ca2+ signaling in pancreatic acinar cells through inhibition of sarco(endo)plasmic reticulum Ca2+-ATPase. Am J Physiol Gastrointest Liver Physiol 287: G1200–G1212, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

