Metallothionein-induced zinc partitioning exacerbates hyperoxic acute lung injury
- PMID: 23275622
- PMCID: PMC3602737
- DOI: 10.1152/ajplung.00243.2012
Metallothionein-induced zinc partitioning exacerbates hyperoxic acute lung injury
Abstract
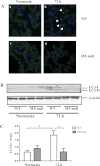
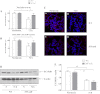
Hypozincemia, with hepatic zinc accumulation at the expense of other organs, occurs in infection, inflammation, and aseptic lung injury. Mechanisms underlying zinc partitioning or its impact on extrahepatic organs are unclear. Here we show that the major zinc-binding protein, metallothionein (MT), is critical for zinc transmigration from lung to liver during hyperoxia and preservation of intrapulmonary zinc during hyperoxia is associated with an injury-resistant phenotype in MT-null mice. Particularly, lung-to-liver zinc ratios decreased in wild-type (WT) and increased significantly in MT-null mice breathing 95% oxygen for 72 h. Compared with female adult WT mice, MT-null mice were significantly protected against hyperoxic lung injury indicated by reduced inflammation and interstitial edema, fewer necrotic changes to distal airway epithelium, and sustained lung function at 72 h hyperoxia. Lungs of MT-null mice showed decreased levels of immunoreactive LC3, an autophagy marker, compared with WT mice. Analysis of superoxide dismutase (SOD) activity in the lungs revealed similar levels of manganese-SOD activity between strains under normoxia and hyperoxia. Lung extracellular SOD activity decreased significantly in both strains at 72 h of hyperoxia, although there was no difference between strains. Copper-zinc-SOD activity was ~4× higher under normoxic conditions in MT-null compared with WT mice but was not affected in either group by hyperoxia. Collectively the data suggest that genetic deletion of MT-I/II in mice is associated with compensatory increase in copper-zinc-SOD activity, prevention of hyperoxia-induced zinc transmigration from lung to liver, and hyperoxia-resistant phenotype strongly associated with differences in zinc homeostasis during hyperoxic acute lung injury.
Figures





Similar articles
-
Aerosolized human extracellular superoxide dismutase prevents hyperoxia-induced lung injury.PLoS One. 2011;6(10):e26870. doi: 10.1371/journal.pone.0026870. Epub 2011 Oct 26. PLoS One. 2011. PMID: 22046389 Free PMC article.
-
Augmented hepatic injury followed by impaired regeneration in metallothionein-I/II knockout mice after treatment with thioacetamide.Toxicol Appl Pharmacol. 2006 Feb 1;210(3):190-9. doi: 10.1016/j.taap.2005.05.007. Epub 2005 Jun 24. Toxicol Appl Pharmacol. 2006. PMID: 15979673
-
Cellular response of antioxidant metalloproteins in Cu/Zn SOD transgenic mice exposed to hyperoxia.Am J Physiol Lung Cell Mol Physiol. 2001 Jul;281(1):L172-82. doi: 10.1152/ajplung.2001.281.1.L172. Am J Physiol Lung Cell Mol Physiol. 2001. PMID: 11404260
-
Transgenic and knockout models for studying the role of lung antioxidant enzymes in defense against hyperoxia.Am J Respir Crit Care Med. 2002 Dec 15;166(12 Pt 2):S51-6. doi: 10.1164/rccm.2206017. Am J Respir Crit Care Med. 2002. PMID: 12471089 Review.
-
Zinc and alcoholic liver disease.Dig Dis. 2010;28(6):745-50. doi: 10.1159/000324282. Epub 2011 Apr 27. Dig Dis. 2010. PMID: 21525759 Free PMC article. Review.
Cited by
-
Tea Bioactive Modulate Innate Immunity: In Perception to COVID-19 Pandemic.Front Immunol. 2020 Oct 28;11:590716. doi: 10.3389/fimmu.2020.590716. eCollection 2020. Front Immunol. 2020. PMID: 33193427 Free PMC article. Review.
-
The Basic Science and Molecular Mechanisms of Lung Injury and Acute Respiratory Distress Syndrome.Int Anesthesiol Clin. 2018 Winter;56(1):1-25. doi: 10.1097/AIA.0000000000000177. Int Anesthesiol Clin. 2018. PMID: 29227309 Free PMC article. Review. No abstract available.
-
Assessment of commitment to healthy daily habits and diets, preventive measures, and beliefs about natural products utilization during COVID-19 pandemic in certain population in Egypt and Saudi Arabia.Pharm Pract (Granada). 2022 Jul-Sep;20(3):2700. doi: 10.18549/PharmPract.2022.3.2700. Epub 2022 Aug 5. Pharm Pract (Granada). 2022. PMID: 36733518 Free PMC article.
References
-
- Abel J, de Ruiter N. Inhibition of hydroxyl-radical-generated DNA degradation by metallothionein. Toxicol Lett 47: 191–196, 1989 - PubMed
-
- Bates JH, Irvin CG. Time dependence of recruitment and derecruitment in the lung: a theoretical model. J Appl Physiol 93: 705–713, 2002 - PubMed
-
- Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem 281: 24085–24089, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

