Kinase drug discovery--what's next in the field?
- PMID: 23276252
- PMCID: PMC4208300
- DOI: 10.1021/cb300610s
Kinase drug discovery--what's next in the field?
Erratum in
- ACS Chem Biol. 2013 Feb 15;8(2):464
Abstract
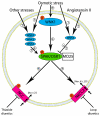
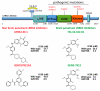
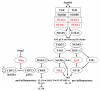
Over the past 15 years protein kinases have become the pharmaceutical industry's most important class of drug target in the field of cancer. Some 20 drugs that target kinases have been approved for clinical use over the past decade, and hundreds more are undergoing clinical trials. However, the recent approval of the first protein kinase inhibitors for the treatment of inflammatory diseases, coupled with an enhanced understanding of the signaling networks that control the immune system, suggests that there will be a surge of interest in this area over the next 10 years. In this connection, we discuss opportunities for targeting protein kinases in the MyD88 signaling network for the development of drugs to treat chronic inflammatory and autoimmune diseases. Activating mutations in protein kinases underlie many other diseases and conditions, and we also discuss why the protein kinases SPAK/OSR1 and LRRK2 have recently become interesting targets for the treatment of hypertension and Parkinson's disease, respectively, and the progress that has been made in developing LRRK2 inhibitors. Finally we suggest that more focus on the identification of inhibitors of kinase activation, rather than kinase activity, may pay dividends in identifying exquisitely specific inhibitors of signal transduction cascades, and we also highlight "pseudo-kinases" as an attractive and unexplored area for drug development that merits much more attention in the years to come.
Figures
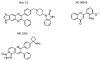
References
-
- Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. - PubMed
-
- Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. - PubMed
-
- Richardson C, Alessi DR. The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1 signalling pathway. J. Cell Sci. 2008;121:3293–3304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G0700656/MRC_/Medical Research Council/United Kingdom
- MC_U127084348/MRC_/Medical Research Council/United Kingdom
- 089698/WT_/Wellcome Trust/United Kingdom
- MC_U127070193/MRC_/Medical Research Council/United Kingdom
- MC_UU_12016/2/MRC_/Medical Research Council/United Kingdom
- MC_UU_12016/11/MRC_/Medical Research Council/United Kingdom
- MR/K000985/1/MRC_/Medical Research Council/United Kingdom
- MC_EX_G0800765/MRC_/Medical Research Council/United Kingdom
- 100294/WT_/Wellcome Trust/United Kingdom
- MC_G1000735/MRC_/Medical Research Council/United Kingdom
- MC_EX_UU_G0800765/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

