Secreted protein kinases
- PMID: 23276407
- PMCID: PMC3582740
- DOI: 10.1016/j.tibs.2012.11.008
Secreted protein kinases
Abstract
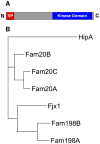
Protein kinases constitute one of the largest gene families and control many aspects of cellular life. In retrospect, the first indication for their existence was reported 130 years ago when the secreted protein, casein, was shown to contain phosphate. Despite its identification as the first phosphoprotein, the responsible kinase has remained obscure. This conundrum was solved with the discovery of a novel family of atypical protein kinases that are secreted and appear to phosphorylate numerous extracellular proteins, including casein. Fam20C, the archetypical member, phosphorylates secreted proteins within Ser-x-Glu/pSer motifs. This discovery has solved a 130-year-old mystery and has shed light on several human disorders of biomineralization.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures



Comment in
-
Secreted protein kinases?Trends Biochem Sci. 2013 Sep;38(9):424-5. doi: 10.1016/j.tibs.2013.06.006. Trends Biochem Sci. 2013. PMID: 23992947 Free PMC article. No abstract available.
-
Response to Wang et al.: Secreted protein kinases?Trends Biochem Sci. 2013 Sep;38(9):425. doi: 10.1016/j.tibs.2013.06.007. Trends Biochem Sci. 2013. PMID: 23992948 No abstract available.
References
-
- Hammarsten O. Zur Frage ob Caseín ein einheitlicher Stoff sei. Hoppe-Seyler’s Zeitschrift für Physiologische Chemie. 1883:227–273.
-
- Lipmann F. Über die Bindung der Phosphorsäre in Phosphorproteinen. I. Biochem Z. 1933;262:3.
-
- Lipmann FA, Levene PA. Serinephosphoric acid obtained on hydrolysis of vitellinic acid. J Biol Chem. 1932;98:109–114.
-
- Burnett G, Kennedy EP. The enzymatic phosphorylation of proteins. J Biol Chem. 1954;211:969–980. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

