On the role of hydrogel structure and degradation in controlling the transport of cell-secreted matrix molecules for engineered cartilage
- PMID: 23276516
- PMCID: PMC3606675
- DOI: 10.1016/j.jmbbm.2012.10.016
On the role of hydrogel structure and degradation in controlling the transport of cell-secreted matrix molecules for engineered cartilage
Abstract
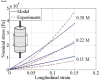
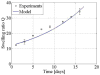
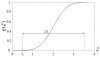
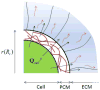
Damage to cartilage caused by injury or disease can lead to pain and loss of mobility, diminishing one's quality of life. Because cartilage has a limited capacity for self-repair, tissue engineering strategies, such as cells encapsulated in synthetic hydrogels, are being investigated as a means to restore the damaged cartilage. However, strategies to date are suboptimal in part because designing degradable hydrogels is complicated by structural and temporal complexities of the gel and evolving tissue along multiple length scales. To address this problem, this study proposes a multi-scale mechanical model using a triphasic formulation (solid, fluid, unbound matrix molecules) based on a single chondrocyte releasing extracellular matrix molecules within a degrading hydrogel. This model describes the key players (cells, proteoglycans, collagen) of the biological system within the hydrogel encompassing different length scales. Two mechanisms are included: temporal changes of bulk properties due to hydrogel degradation, and matrix transport. Numerical results demonstrate that the temporal change of bulk properties is a decisive factor in the diffusion of unbound matrix molecules through the hydrogel. Transport of matrix molecules in the hydrogel contributes both to the development of the pericellular matrix and the extracellular matrix and is dependent on the relative size of matrix molecules and the hydrogel mesh. The numerical results also demonstrate that osmotic pressure, which leads to changes in mesh size, is a key parameter for achieving a larger diffusivity for matrix molecules in the hydrogel. The numerical model is confirmed with experimental results of matrix synthesis by chondrocytes in biodegradable poly(ethylene glycol)-based hydrogels. This model may ultimately be used to predict key hydrogel design parameters towards achieving optimal cartilage growth.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures











References
-
- Ateshian GA, Kim JJ, Grelsamer RP, Mow VC, Warden WH. Finite deformation of bovine material properties cartilage compression. 1997;30(97) - PubMed
-
- Bell CL, Peppas NA. Biomedical Membranes from Hydrogels and Interpolymer Complexes. Polymer. 1995;122
-
- Bryant SJ, Anseth KS. Controlling the spatial distribution of ECM components in degradable PEG hydrogels for tissue engineering cartilage. Journal of biomedical materials research Part A. 2003;64(1):70–9. - PubMed
-
- Bryant SJ, Bender RJ, Durand KL, Anseth KS. Encapsulating chondrocytes in degrading PEG hydrogels with high modulus: engineering gel structural changes to facilitate cartilaginous tissue production. Biotechnology and bioengineering. 2004a;86(7):747–55. - PubMed
-
- Bryant SJ, Chowdhury TT, Lee DA, Bader DL, Anseth KS. Crosslinking density influences chondrocyte metabolism in dynamically loaded photocrosslinked poly(ethylene glycol) hydrogels. Annals of biomedical engineering. 2004;32(3):407–17. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

