(R)-β-lysine-modified elongation factor P functions in translation elongation
- PMID: 23277358
- PMCID: PMC3567691
- DOI: 10.1074/jbc.M112.438879
(R)-β-lysine-modified elongation factor P functions in translation elongation
Abstract
Post-translational modification of bacterial elongation factor P (EF-P) with (R)-β-lysine at a conserved lysine residue activates the protein in vivo and increases puromycin reactivity of the ribosome in vitro. The additional hydroxylation of EF-P at the same lysine residue by the YfcM protein has also recently been described. The roles of modified and unmodified EF-P during different steps in translation, and how this correlates to its physiological role in the cell, have recently been linked to the synthesis of polyproline stretches in proteins. Polysome analysis indicated that EF-P functions in translation elongation, rather than initiation as proposed previously. This was further supported by the inability of EF-P to enhance the rate of formation of fMet-Lys or fMet-Phe, indicating that the role of EF-P is not to specifically stimulate formation of the first peptide bond. Investigation of hydroxyl-(β)-lysyl-EF-P showed 30% increased puromycin reactivity but no differences in dipeptide synthesis rates when compared with the β-lysylated form. Unlike disruption of the other genes required for EF-P modification, deletion of yfcM had no phenotypic consequences in Salmonella. Taken together, our findings indicate that EF-P functions in translation elongation, a role critically dependent on post-translational β-lysylation but not hydroxylation.
Figures


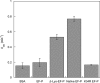

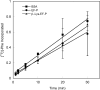
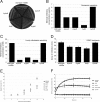
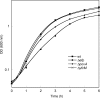
 ) E. coli strains. OD, optical density.
) E. coli strains. OD, optical density.References
-
- Aoki H., Xu J., Emili A., Chosay J. G., Golshani A., Ganoza M. C. (2008) Interactions of elongation factor EF-P with the Escherichia coli ribosome. FEBS J. 275, 671–681 - PubMed
-
- Doerfel L. K., Wohlgemuth I., Kothe C., Peske F., Urlaub H., Rodnina M. V. (2013) EF-P is essential for rapid synthesis of proteins containing consecutive proline residues. Science 339, 85–88 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

