ATAD5 regulates the lifespan of DNA replication factories by modulating PCNA level on the chromatin
- PMID: 23277426
- PMCID: PMC3542800
- DOI: 10.1083/jcb.201206084
ATAD5 regulates the lifespan of DNA replication factories by modulating PCNA level on the chromatin
Abstract
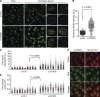
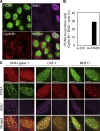
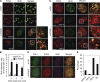
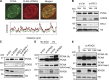
Temporal and spatial regulation of the replication factory is important for efficient DNA replication. However, the underlying molecular mechanisms are not well understood. Here, we report that ATAD5 regulates the lifespan of replication factories. Reduced expression of ATAD5 extended the lifespan of replication factories by retaining proliferating cell nuclear antigen (PCNA) and other replisome proteins on the chromatin during and even after DNA synthesis. This led to an increase of inactive replication factories with an accumulation of replisome proteins. Consequently, the overall replication rate was decreased, which resulted in the delay of S-phase progression. Prevalent detection of PCNA foci in G2 phase cells after ATAD5 depletion suggests that defects in the disassembly of replication factories persist after S phase is complete. ATAD5-mediated regulation of the replication factory and PCNA required an intact ATAD5 ATPase domain. Taken together, our data imply that ATAD5 regulates the cycle of DNA replication factories, probably through its PCNA-unloading activity.
Figures




References
-
- Bell D.W., Sikdar N., Lee K.Y., Price J.C., Chatterjee R., Park H.D., Fox J., Ishiai M., Rudd M.L., Pollock L.M., et al. ; NISC Comparative Sequencing Program 2011. Predisposition to cancer caused by genetic and functional defects of mammalian Atad5. PLoS Genet. 7:e1002245 10.1371/journal.pgen.1002245 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

