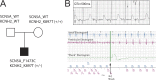
Induced pluripotent stem cells used to reveal drug actions in a long QT syndrome family with complex genetics
- PMID: 23277474
- PMCID: PMC3536519
- DOI: 10.1085/jgp.201210899
Induced pluripotent stem cells used to reveal drug actions in a long QT syndrome family with complex genetics
Abstract
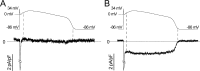
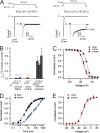
Understanding the basis for differential responses to drug therapies remains a challenge despite advances in genetics and genomics. Induced pluripotent stem cells (iPSCs) offer an unprecedented opportunity to investigate the pharmacology of disease processes in therapeutically and genetically relevant primary cell types in vitro and to interweave clinical and basic molecular data. We report here the derivation of iPSCs from a long QT syndrome patient with complex genetics. The proband was found to have a de novo SCN5A LQT-3 mutation (F1473C) and a polymorphism (K897T) in KCNH2, the gene for LQT-2. Analysis of the biophysics and molecular pharmacology of ion channels expressed in cardiomyocytes (CMs) differentiated from these iPSCs (iPSC-CMs) demonstrates a primary LQT-3 (Na(+) channel) defect responsible for the arrhythmias not influenced by the KCNH2 polymorphism. The F1473C mutation occurs in the channel inactivation gate and enhances late Na(+) channel current (I(NaL)) that is carried by channels that fail to inactivate completely and conduct increased inward current during prolonged depolarization, resulting in delayed repolarization, a prolonged QT interval, and increased risk of fatal arrhythmia. We find a very pronounced rate dependence of I(NaL) such that increasing the pacing rate markedly reduces I(NaL) and, in addition, increases its inhibition by the Na(+) channel blocker mexiletine. These rate-dependent properties and drug interactions, unique to the proband's iPSC-CMs, correlate with improved management of arrhythmias in the patient and provide support for this approach in developing patient-specific clinical regimens.
Figures






Comment in
-
On optimizing therapies, Orai, and ORNs.J Gen Physiol. 2013 Feb;141(2):149-50. doi: 10.1085/jgp.201310958. J Gen Physiol. 2013. PMID: 23359279 Free PMC article. No abstract available.
References
-
- Bankston J.R., Sampson K.J., Kateriya S., Glaaser I.W., Malito D.L., Chung W.K., Kass R.S. 2007a. A novel LQT-3 mutation disrupts an inactivation gate complex with distinct rate-dependent phenotypic consequences. Channels (Austin). 1:273–280 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

