Guideline-discordant androgen deprivation therapy in localized prostate cancer: patterns of use in the medicare population and cost implications
- PMID: 23277483
- PMCID: PMC3629897
- DOI: 10.1093/annonc/mds618
Guideline-discordant androgen deprivation therapy in localized prostate cancer: patterns of use in the medicare population and cost implications
Abstract
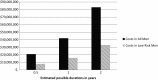
Background Androgen deprivation therapy (ADT) in localized prostate cancer improves overall survival and is recommended by National Comprehensive Cancer Network guidelines in certain situations. However, ADT is without benefit in other situations and can actually cause harm. This study examines recent trends in the ADT use and quantifies the cost of guideline-discordant ADT. Patients and methods Patients, aged 66-80 years, in the Surveillance Epidemiology and End Results-Medicare database with non-metastatic prostate cancer diagnosed between 2004 and 2007 were included for analysis. Prostate-specific antigen, Gleason score, and stage were used to define D'Amico risk categories. Logistic regression was used to examine factors associated with guideline-discordant ADT. Annual direct cost was estimated using 2011 Medicare reimbursement for ADT. Results Of 28 654 men included, 12.4% received guideline-discordant ADT. In low-risk patients, 14.9% received discordant ADT, mostly due to simultaneous ADT with radiation. Discordant use was seen in 7.3% of intermediate and 14.9% of high-risk patients, mostly from ADT as primary therapy. The odds of receiving guideline-discordant ADT decreased over time (2007 versus 2004; OR 0.69; 95% CI 0.62-0.76). The estimated annual direct cost from discordant ADT is $42 000 000. Conclusion Approximately one in eight patients received ADT discordant with published guidelines. Elimination of discordant use would result in substantial savings.
Figures
References
-
- Bethesda, MD: ational Cancer Institute; SEER Cancer Statistics Review, 1975–2008. http://seer.cancer.gov/csr/1975_2008/ 9 December 2011, ccessed)
-
- American Cancer Society. Cancer Facts & Figures 2011 . http://www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/can.... 9 December 2011, ccessed)
-
- D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–974. - PubMed
-
- Mohler JL. The 2010 NCCN clinical practice guidelines in oncology on prostate cancer. J Natl Compr Canc Netw. 2010;8(2):145. - PubMed
-
- Cooperberg MR, Carroll PR, Klotz L. Active surveillance for prostate cancer: progress and promise. J Clin Oncol. 2011;29(27):3669–3676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

