Centrosome positioning in vertebrate development
- PMID: 23277534
- PMCID: PMC3533386
- DOI: 10.1242/jcs.038083
Centrosome positioning in vertebrate development
Abstract
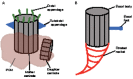
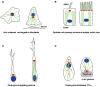
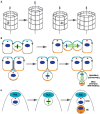
The centrosome, a major organizer of microtubules, has important functions in regulating cell shape, polarity, cilia formation and intracellular transport as well as the position of cellular structures, including the mitotic spindle. By means of these activities, centrosomes have important roles during animal development by regulating polarized cell behaviors, such as cell migration or neurite outgrowth, as well as mitotic spindle orientation. In recent years, the pace of discovery regarding the structure and composition of centrosomes has continuously accelerated. At the same time, functional studies have revealed the importance of centrosomes in controlling both morphogenesis and cell fate decision during tissue and organ development. Here, we review examples of centrosome and centriole positioning with a particular emphasis on vertebrate developmental systems, and discuss the roles of centrosome positioning, the cues that determine positioning and the mechanisms by which centrosomes respond to these cues. The studies reviewed here suggest that centrosome functions extend to the development of tissues and organs in vertebrates.
Figures
Similar articles
-
Positioning centrioles and centrosomes.J Cell Biol. 2024 Apr 1;223(4):e202311140. doi: 10.1083/jcb.202311140. Epub 2024 Mar 21. J Cell Biol. 2024. PMID: 38512059 Free PMC article. Review.
-
Centrosome positioning in polarized cells: common themes and variations.Exp Cell Res. 2014 Nov 1;328(2):240-8. doi: 10.1016/j.yexcr.2014.09.004. Epub 2014 Sep 8. Exp Cell Res. 2014. PMID: 25218948 Review.
-
The centrosome and bipolar spindle assembly: does one have anything to do with the other?Cell Cycle. 2011 Nov 15;10(22):3841-8. doi: 10.4161/cc.10.22.18293. Epub 2011 Nov 15. Cell Cycle. 2011. PMID: 22071626 Free PMC article.
-
[Centrosome as "a brain" of an animal cell].Tsitologiia. 2008;50(1):5-17. Tsitologiia. 2008. PMID: 18409364 Review. Russian.
-
New frontiers: discovering cilia-independent functions of cilia proteins.EMBO Rep. 2015 Oct;16(10):1275-87. doi: 10.15252/embr.201540632. Epub 2015 Sep 9. EMBO Rep. 2015. PMID: 26358956 Free PMC article. Review.
Cited by
-
A role for the centrosome in regulating the rate of neuronal efferocytosis by microglia in vivo.Elife. 2022 Nov 18;11:e82094. doi: 10.7554/eLife.82094. Elife. 2022. PMID: 36398880 Free PMC article.
-
Excess centrosomes perturb dynamic endothelial cell repolarization during blood vessel formation.Mol Biol Cell. 2016 Jun 15;27(12):1911-20. doi: 10.1091/mbc.E15-09-0645. Epub 2016 Apr 20. Mol Biol Cell. 2016. PMID: 27099371 Free PMC article.
-
Intraflagellar transport protein 74 is essential for spermatogenesis and male fertility in mice†.Biol Reprod. 2019 Jul 1;101(1):188-199. doi: 10.1093/biolre/ioz071. Biol Reprod. 2019. PMID: 31004481 Free PMC article.
-
Epithelial homeostasis.Curr Biol. 2014 Sep 8;24(17):R815-25. doi: 10.1016/j.cub.2014.06.068. Curr Biol. 2014. PMID: 25202877 Free PMC article. Review.
-
Centering and Shifting of Centrosomes in Cells.Cells. 2020 May 29;9(6):1351. doi: 10.3390/cells9061351. Cells. 2020. PMID: 32485978 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

