Impact of seawater acidification on pH at the tissue-skeleton interface and calcification in reef corals
- PMID: 23277567
- PMCID: PMC3562847
- DOI: 10.1073/pnas.1216153110
Impact of seawater acidification on pH at the tissue-skeleton interface and calcification in reef corals
Abstract
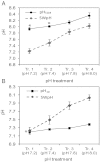
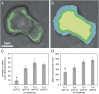
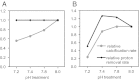
Insight into the response of reef corals and other major marine calcifiers to ocean acidification is limited by a lack of knowledge about how seawater pH and carbonate chemistry impact the physiological processes that drive biomineralization. Ocean acidification is proposed to reduce calcification rates in corals by causing declines in internal pH at the calcifying tissue-skeleton interface where biomineralization takes place. Here, we performed an in vivo study on how partial-pressure CO(2)-driven seawater acidification impacts intracellular pH in coral calcifying cells and extracellular pH in the fluid at the tissue-skeleton interface [subcalicoblastic medium (SCM)] in the coral Stylophora pistillata. We also measured calcification in corals grown under the same conditions of seawater acidification by measuring lateral growth of colonies and growth of aragonite crystals under the calcifying tissue. Our findings confirm that seawater acidification decreases pH of the SCM, but this decrease is gradual relative to the surrounding seawater, leading to an increasing pH gradient between the SCM and seawater. Reductions in calcification rate, both at the level of crystals and whole colonies, were only observed in our lowest pH treatment when pH was significantly depressed in the calcifying cells in addition to the SCM. Overall, our findings suggest that reef corals may mitigate the effects of seawater acidification by regulating pH in the SCM, but they also highlight the role of calcifying cell pH homeostasis in determining the response of reef corals to changes in external seawater pH and carbonate chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Comment in
-
Coral calcification feels the acid.Proc Natl Acad Sci U S A. 2013 Jan 29;110(5):1567-8. doi: 10.1073/pnas.1221308110. Epub 2013 Jan 17. Proc Natl Acad Sci U S A. 2013. PMID: 23329329 Free PMC article. No abstract available.
References
-
- Kroeker KJ, Kordas RL, Crim RN, Singh GG. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol Lett. 2010;13(11):1419–1434. - PubMed
-
- Rodolfo-Metalpa R, et al. Coral and mollusc resistance to ocean acidification adversely affected by warming. Nat Clim Chang. 2011;1(6):308–312.
-
- Pandolfi JM, Connolly SR, Marshall DJ, Cohen AL. Projecting coral reef futures under global warming and ocean acidification. Science. 2011;333(6041):418–422. - PubMed
-
- Ries J, Cohen A, McCorkle D. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology. 2009;37(12):1131–1134.
-
- Weis VM, Allemand D. Physiology. What determines coral health? Science. 2009;324(5931):1153–1155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

