Robust and tunable circadian rhythms from differentially sensitive catalytic domains
- PMID: 23277568
- PMCID: PMC3549141
- DOI: 10.1073/pnas.1212113110
Robust and tunable circadian rhythms from differentially sensitive catalytic domains
Abstract
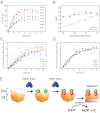
Circadian clocks are ubiquitous biological oscillators that coordinate an organism's behavior with the daily cycling of the external environment. To ensure synchronization with the environment, the period of the clock must be maintained near 24 h even as amplitude and phase are altered by input signaling. We show that, in a reconstituted circadian system from cyanobacteria, these conflicting requirements are satisfied by distinct functions for two domains of the central clock protein KaiC: the C-terminal autokinase domain integrates input signals through the ATP/ADP ratio, and the slow N-terminal ATPase acts as an input-independent timer. We find that phosphorylation in the C-terminal domain followed by an ATPase cycle in the N-terminal domain is required to form the inhibitory KaiB•KaiC complexes that drive the dynamics of the clock. We present a mathematical model in which this ATPase-mediated delay in negative feedback gives rise to a compensatory mechanism that allows a tunable phase and amplitude while ensuring a robust circadian period.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Winfree AT. The Geometry of Biological Time. 2nd Ed. New York: Springer; 2000.
-
- Martino TA, et al. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am J Physiol Regul Integr Comp Physiol. 2008;294(5):R1675–R1683. - PubMed
-
- Nakajima M, et al. Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. Science. 2005;308(5720):414–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

