Mechanism of proton-coupled quinone reduction in Photosystem II
- PMID: 23277574
- PMCID: PMC3549079
- DOI: 10.1073/pnas.1212957110
Mechanism of proton-coupled quinone reduction in Photosystem II
Abstract
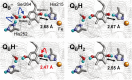
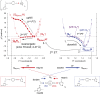
Photosystem II uses light to drive water oxidation and plastoquinone (PQ) reduction. PQ reduction involves two PQ cofactors, Q(A) and Q(B), working in series. Q(A) is a one-electron carrier, whereas Q(B) undergoes sequential reduction and protonation to form Q(B)H(2). Q(B)H(2) exchanges with PQ from the pool in the membrane. Based on the atomic coordinates of the Photosystem II crystal structure, we analyzed the proton transfer (PT) energetics adopting a quantum mechanical/molecular mechanical approach. The potential-energy profile suggests that the initial PT to Q(B)(•-) occurs from the protonated, D1-His252 to Q(B)(•)(-) via D1-Ser264. The second PT is likely to occur from D1-His215 to Q(B)H(-) via an H-bond with an energy profile with a single well, resulting in the formation of Q(B)H(2) and the D1-His215 anion. The pathway for reprotonation of D1-His215(-) may involve bicarbonate, D1-Tyr246 and water in the Q(B) site. Formate ligation to Fe(2+) did not significantly affect the protonation of reduced Q(B), suggesting that formate inhibits Q(B)H(2) release rather than its formation. The presence of carbonate rather than bicarbonate seems unlikely because the calculations showed that this greatly perturbed the potential of the nonheme iron, stabilizing the Fe(3+) state in the presence of Q(B)(•-), a situation not encountered experimentally. H-bonding from D1-Tyr246 and D2-Tyr244 to the bicarbonate ligand of the nonheme iron contributes to the stability of the semiquinones. A detailed mechanistic model for Q(B) reduction is presented.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Control of quinone redox potentials in photosystem II: Electron transfer and photoprotection.J Am Chem Soc. 2005 Oct 26;127(42):14714-20. doi: 10.1021/ja052567r. J Am Chem Soc. 2005. PMID: 16231925
-
Oxidation of the non-heme iron complex in photosystem II.Biochemistry. 2005 Nov 15;44(45):14772-83. doi: 10.1021/bi051099v. Biochemistry. 2005. PMID: 16274225
-
How does the QB site influence propagate to the QA site in photosystem II?Biochemistry. 2011 Jun 21;50(24):5436-42. doi: 10.1021/bi102023x. Epub 2011 May 23. Biochemistry. 2011. PMID: 21585193
-
Coupling of electron transfer to proton uptake at the Q(B) site of the bacterial reaction center: a perspective from FTIR difference spectroscopy.Biochim Biophys Acta. 2008 Oct;1777(10):1229-48. doi: 10.1016/j.bbabio.2008.06.012. Epub 2008 Jul 11. Biochim Biophys Acta. 2008. PMID: 18671937 Review.
-
Light-induced quinone reduction in photosystem II.Biochim Biophys Acta. 2012 Jan;1817(1):44-65. doi: 10.1016/j.bbabio.2011.05.021. Epub 2011 Jun 1. Biochim Biophys Acta. 2012. PMID: 21679684 Review.
Cited by
-
Proton-mediated photoprotection mechanism in photosystem II.Front Plant Sci. 2022 Sep 7;13:934736. doi: 10.3389/fpls.2022.934736. eCollection 2022. Front Plant Sci. 2022. PMID: 36161009 Free PMC article.
-
Conformational control over proton-coupled electron transfer in metalloenzymes.Nat Rev Chem. 2024 Oct;8(10):762-775. doi: 10.1038/s41570-024-00646-7. Epub 2024 Sep 2. Nat Rev Chem. 2024. PMID: 39223400 Free PMC article. Review.
-
Review on NAD(P)H dehydrogenase quinone 1 (NQO1) pathway.Mol Biol Rep. 2022 Sep;49(9):8907-8924. doi: 10.1007/s11033-022-07369-2. Epub 2022 Mar 28. Mol Biol Rep. 2022. PMID: 35347544 Review.
-
The plastoquinol-plastoquinone exchange mechanism in photosystem II: insight from molecular dynamics simulations.Photosynth Res. 2017 Jan;131(1):15-30. doi: 10.1007/s11120-016-0292-4. Epub 2016 Jul 4. Photosynth Res. 2017. PMID: 27376842
-
Conformational Changes and H-Bond Rearrangements during Quinone Release in Photosystem II.Biochemistry. 2022 Sep 6;61(17):1836-1843. doi: 10.1021/acs.biochem.2c00324. Epub 2022 Aug 1. Biochemistry. 2022. PMID: 35914244 Free PMC article.
References
-
- Diner BA, Rappaport F. Structure, dynamics, and energetics of the primary photochemistry of photosystem II of oxygenic photosynthesis. Annu Rev Plant Biol. 2002;53:551–580. - PubMed
-
- Renger G, Renger T. Photosystem II: The machinery of photosynthetic water splitting. Photosynth Res. 2008;98(1-3):53–80. - PubMed
-
- Holzwarth AR. Ultrafast primary reactions in the photosystems of oxygen evolving organisms. In: Braun M, Gilch P, Zinth W, editors. Ultrashort Laser Pulses in Biology and Medicine, Biological and Medical Physics, Biomedical Engineering. Dordrecht, The Netherlands: Springer; 2008. pp. 141–164.
-
- Cardona T, Sedoud A, Cox N, Rutherford AW. Charge separation in photosystem II: A comparative and evolutionary overview. Biochim Biophys Acta. 2012;1817(1):26–43. - PubMed
-
- Müh F, Glöckner C, Hellmich J, Zouni A. Light-induced quinone reduction in photosystem II. Biochim Biophys Acta. 2012;1817(1):44–65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

