Hydroxylamine as an intermediate in ammonia oxidation by globally abundant marine archaea
- PMID: 23277575
- PMCID: PMC3549078
- DOI: 10.1073/pnas.1214272110
Hydroxylamine as an intermediate in ammonia oxidation by globally abundant marine archaea
Abstract
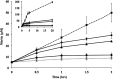
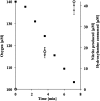
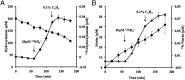
The ammonia-oxidizing archaea have recently been recognized as a significant component of many microbial communities in the biosphere. Although the overall stoichiometry of archaeal chemoautotrophic growth via ammonia (NH(3)) oxidation to nitrite (NO(2)(-)) is superficially similar to the ammonia-oxidizing bacteria, genome sequence analyses point to a completely unique biochemistry. The only genomic signature linking the bacterial and archaeal biochemistries of NH(3) oxidation is a highly divergent homolog of the ammonia monooxygenase (AMO). Although the presumptive product of the putative AMO is hydroxylamine (NH(2)OH), the absence of genes encoding a recognizable ammonia-oxidizing bacteria-like hydroxylamine oxidoreductase complex necessitates either a novel enzyme for the oxidation of NH(2)OH or an initial oxidation product other than NH(2)OH. We now show through combined physiological and stable isotope tracer analyses that NH(2)OH is both produced and consumed during the oxidation of NH(3) to NO(2)(-) by Nitrosopumilus maritimus, that consumption is coupled to energy conversion, and that NH(2)OH is the most probable product of the archaeal AMO homolog. Thus, despite their deep phylogenetic divergence, initial oxidation of NH(3) by bacteria and archaea appears mechanistically similar. They however diverge biochemically at the point of oxidation of NH(2)OH, the archaea possibly catalyzing NH(2)OH oxidation using a novel enzyme complex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
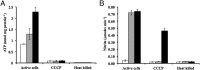
Similar articles
-
Inhibition of Ammonia Monooxygenase from Ammonia-Oxidizing Archaea by Linear and Aromatic Alkynes.Appl Environ Microbiol. 2020 Apr 17;86(9):e02388-19. doi: 10.1128/AEM.02388-19. Print 2020 Apr 17. Appl Environ Microbiol. 2020. PMID: 32086308 Free PMC article.
-
Enrichment and characterization of ammonia-oxidizing archaea from the open ocean: phylogeny, physiology and stable isotope fractionation.ISME J. 2011 Nov;5(11):1796-808. doi: 10.1038/ismej.2011.58. Epub 2011 May 12. ISME J. 2011. PMID: 21562601 Free PMC article.
-
Molecular biology and biochemistry of ammonia oxidation by Nitrosomonas europaea.Arch Microbiol. 2002 Oct;178(4):250-5. doi: 10.1007/s00203-002-0452-0. Epub 2002 Jun 27. Arch Microbiol. 2002. PMID: 12209257 Review.
-
Nitrogen metabolism and kinetics of ammonia-oxidizing archaea.Methods Enzymol. 2011;496:465-87. doi: 10.1016/B978-0-12-386489-5.00019-1. Methods Enzymol. 2011. PMID: 21514476 Review.
-
High-rate, high-yield production of methanol by ammonia-oxidizing bacteria.Environ Sci Technol. 2013 Apr 2;47(7):3167-73. doi: 10.1021/es3042912. Epub 2013 Mar 21. Environ Sci Technol. 2013. PMID: 23473425
Cited by
-
Ammonia-oxidizing archaea use the most energy-efficient aerobic pathway for CO2 fixation.Proc Natl Acad Sci U S A. 2014 Jun 3;111(22):8239-44. doi: 10.1073/pnas.1402028111. Epub 2014 May 19. Proc Natl Acad Sci U S A. 2014. PMID: 24843170 Free PMC article.
-
Oxygen at nanomolar levels reversibly suppresses process rates and gene expression in anammox and denitrification in the oxygen minimum zone off northern Chile.mBio. 2014 Oct 28;5(6):e01966. doi: 10.1128/mBio.01966-14. mBio. 2014. PMID: 25352619 Free PMC article.
-
Pathways and key intermediates required for obligate aerobic ammonia-dependent chemolithotrophy in bacteria and Thaumarchaeota.ISME J. 2016 Aug;10(8):1836-45. doi: 10.1038/ismej.2016.2. Epub 2016 Feb 16. ISME J. 2016. PMID: 26882267 Free PMC article.
-
Analysis of biomass productivity and physiology of Nitrososphaera viennensis grown in continuous culture.Front Microbiol. 2023 Feb 16;14:1076342. doi: 10.3389/fmicb.2023.1076342. eCollection 2023. Front Microbiol. 2023. PMID: 36876066 Free PMC article.
-
Thaumarchaea Genome Sequences from a High Arctic Active Layer.Microbiol Resour Announc. 2020 May 21;9(21):e00326-20. doi: 10.1128/MRA.00326-20. Microbiol Resour Announc. 2020. PMID: 32439668 Free PMC article.
References
-
- de la Torre JR, Walker CB, Ingalls AE, Könneke M, Stahl DA. Cultivation of a thermophilic ammonia oxidizing archaeon synthesizing crenarchaeol. Environ Microbiol. 2008;10(3):810–818. - PubMed
-
- Könneke M, et al. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature. 2005;437(7058):543–546. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

