Involvement of distinct arrestin-1 elements in binding to different functional forms of rhodopsin
- PMID: 23277586
- PMCID: PMC3549108
- DOI: 10.1073/pnas.1215176110
Involvement of distinct arrestin-1 elements in binding to different functional forms of rhodopsin
Abstract
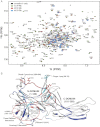
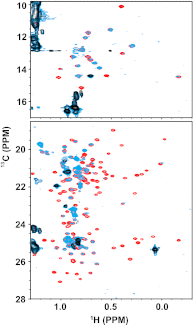
Solution NMR spectroscopy of labeled arrestin-1 was used to explore its interactions with dark-state phosphorylated rhodopsin (P-Rh), phosphorylated opsin (P-opsin), unphosphorylated light-activated rhodopsin (Rh*), and phosphorylated light-activated rhodopsin (P-Rh*). Distinct sets of arrestin-1 elements were seen to be engaged by Rh* and inactive P-Rh, which induced conformational changes that differed from those triggered by binding of P-Rh*. Although arrestin-1 affinity for Rh* was seen to be low (K(D) > 150 μM), its affinity for P-Rh (K(D) ~80 μM) was comparable to the concentration of active monomeric arrestin-1 in the outer segment, suggesting that P-Rh generated by high-gain phosphorylation is occupied by arrestin-1 under physiological conditions and will not signal upon photo-activation. Arrestin-1 was seen to bind P-Rh* and P-opsin with fairly high affinity (K(D) of~50 and 800 nM, respectively), implying that arrestin-1 dissociation is triggered only upon P-opsin regeneration with 11-cis-retinal, precluding noise generated by opsin activity. Based on their observed affinity for arrestin-1, P-opsin and inactive P-Rh very likely affect the physiological monomer-dimer-tetramer equilibrium of arrestin-1, and should therefore be taken into account when modeling photoreceptor function. The data also suggested that complex formation with either P-Rh* or P-opsin results in a global transition in the conformation of arrestin-1, possibly to a dynamic molten globule-like structure. We hypothesize that this transition contributes to the mechanism that triggers preferential interactions of several signaling proteins with receptor-activated arrestins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Constitutively active rhodopsin mutants causing night blindness are effectively phosphorylated by GRKs but differ in arrestin-1 binding.Cell Signal. 2013 Nov;25(11):2155-62. doi: 10.1016/j.cellsig.2013.07.009. Epub 2013 Jul 17. Cell Signal. 2013. PMID: 23872075 Free PMC article.
-
Transition of arrestin into the active receptor-binding state requires an extended interdomain hinge.J Biol Chem. 2002 Nov 15;277(46):43961-7. doi: 10.1074/jbc.M206951200. Epub 2002 Sep 4. J Biol Chem. 2002. PMID: 12215448
-
The arrestin-bound conformation and dynamics of the phosphorylated carboxy-terminal region of rhodopsin.FEBS Lett. 2004 Apr 30;564(3):307-11. doi: 10.1016/S0014-5793(04)00226-1. FEBS Lett. 2004. PMID: 15111114
-
Structure and self-association of Arrestin-1.J Struct Biol. 2025 Mar;217(1):108173. doi: 10.1016/j.jsb.2025.108173. Epub 2025 Jan 27. J Struct Biol. 2025. PMID: 39880147 Review.
-
Structural Basis of Arrestin Selectivity for Active Phosphorylated G Protein-Coupled Receptors.Int J Mol Sci. 2021 Nov 19;22(22):12481. doi: 10.3390/ijms222212481. Int J Mol Sci. 2021. PMID: 34830362 Free PMC article. Review.
Cited by
-
Extensive shape shifting underlies functional versatility of arrestins.Curr Opin Cell Biol. 2014 Apr;27:1-9. doi: 10.1016/j.ceb.2013.10.007. Epub 2013 Nov 16. Curr Opin Cell Biol. 2014. PMID: 24680424 Free PMC article. Review.
-
ACKR3-arrestin2/3 complexes reveal molecular consequences of GRK-dependent barcoding.bioRxiv [Preprint]. 2023 Jul 19:2023.07.18.549504. doi: 10.1101/2023.07.18.549504. bioRxiv. 2023. PMID: 37502840 Free PMC article. Preprint.
-
Crystal structure of a common GPCR-binding interface for G protein and arrestin.Nat Commun. 2014 Sep 10;5:4801. doi: 10.1038/ncomms5801. Nat Commun. 2014. PMID: 25205354 Free PMC article.
-
Expression of Untagged Arrestins in E. coli and Their Purification.Curr Protoc. 2023 Sep;3(9):e832. doi: 10.1002/cpz1.832. Curr Protoc. 2023. PMID: 37671938 Free PMC article.
-
β-Arrestin2 oligomers impair the clearance of pathological tau and increase tau aggregates.Proc Natl Acad Sci U S A. 2020 Mar 3;117(9):5006-5015. doi: 10.1073/pnas.1917194117. Epub 2020 Feb 18. Proc Natl Acad Sci U S A. 2020. PMID: 32071246 Free PMC article.
References
-
- Kühn H. Light-regulated binding of rhodopsin kinase and other proteins to cattle photoreceptor membranes. Biochemistry. 1978;17(21):4389–4395. - PubMed
-
- Wilden U. Duration and amplitude of the light-induced cGMP hydrolysis in vertebrate photoreceptors are regulated by multiple phosphorylation of rhodopsin and by arrestin binding. Biochemistry. 1995;34(4):1446–1454. - PubMed
-
- Krupnick JG, Gurevich VV, Benovic JL. Mechanism of quenching of phototransduction. Binding competition between arrestin and transducin for phosphorhodopsin. J Biol Chem. 1997;272(29):18125–18131. - PubMed
-
- Gurevich VV, Benovic JL. Cell-free expression of visual arrestin. Truncation mutagenesis identifies multiple domains involved in rhodopsin interaction. J Biol Chem. 1992;267(30):21919–21923. - PubMed
-
- Gurevich VV, Benovic JL. Visual arrestin interaction with rhodopsin. Sequential multisite binding ensures strict selectivity toward light-activated phosphorylated rhodopsin. J Biol Chem. 1993;268(16):11628–11638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

