Cost effectiveness of personalized therapy for first-line treatment of stage IV and recurrent incurable adenocarcinoma of the lung
- PMID: 23277762
- PMCID: PMC3439225
- DOI: 10.1200/JOP.2011.000502
Cost effectiveness of personalized therapy for first-line treatment of stage IV and recurrent incurable adenocarcinoma of the lung
Abstract
Purpose: Patients with epidermal growth factor receptor (EGFR) mutation-positive stage IV adenocarcinoma have improved survival with tyrosine kinase inhibitor (TKI) treatments, but the cost effectiveness of personalized first-line therapy using EGFR mutation testing is unknown.
Methods: We created a decision analytic model comparing the costs and effects of platinum combination chemotherapy with personalized therapy in which patients with EGFR mutation-positive tumors were treated with erlotinib. We used two testing strategies: testing only those with tissue available and performing a repeat biopsy if tissue was not available versus three nontargeted chemotherapy regimens (ie, carboplatin and paclitaxel; carboplatin and pemetrexed; and carboplatin, pemetrexed, and bevacizumab).
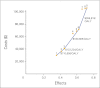
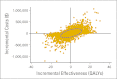
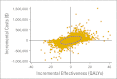
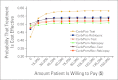
Results: Compared with a carboplatin plus paclitaxel regimen, targeted therapy based on testing available tissue yielded an incremental cost-effectiveness ratio (ICER) of $110,644 per quality-adjusted life year (QALY), and the rebiopsy strategy yielded an ICER of $122,219 per QALY. Probabilistic sensitivity analysis revealed substantial uncertainty around these point estimates. With a willingness to pay of $100,000 per QALY, the testing strategy was cost effective 58% of the time, and the rebiopsy strategy was cost effective 54% of the time. Personalized therapy with an EGFR TKI was more favorable when the nontargeted chemotherapy regimen was more expensive. Compared with carboplatin, pemetrexed, and bevacizumab, ICERs were $25,547 per QALY for the testing strategy and $44,036 per QALY for the rebiopsy strategy.
Conclusion: Although specific clinical circumstances should guide therapy, our cost-effectiveness analysis supports the strategy of testing for EGFR mutations in patients with stage IV or recurrent adenocarcinoma of the lung, rebiopsying patients if insufficient tissue is available for testing, and treating patients with EGFR mutations with erlotinib as first-line therapy.
Figures
Similar articles
-
Cost-Effectiveness and Value of Information of Erlotinib, Afatinib, and Cisplatin-Pemetrexed for First-Line Treatment of Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer in the United States.Value Health. 2015 Sep;18(6):774-82. doi: 10.1016/j.jval.2015.04.008. Epub 2015 Jun 22. Value Health. 2015. PMID: 26409604
-
Cost-Effectiveness of an Individualized First-Line Treatment Strategy Offering Erlotinib Based on EGFR Mutation Testing in Advanced Lung Adenocarcinoma Patients in Germany.Pharmacoeconomics. 2015 Nov;33(11):1215-28. doi: 10.1007/s40273-015-0305-8. Pharmacoeconomics. 2015. PMID: 26081300
-
Cost-effectiveness of Osimertinib as a Second-line Treatment in Patients With EGFR-mutated Advanced Non-Small Cell Lung Cancer in China.Clin Ther. 2019 Nov;41(11):2308-2320.e11. doi: 10.1016/j.clinthera.2019.09.008. Epub 2019 Oct 10. Clin Ther. 2019. PMID: 31607559
-
Gefitinib for the first-line treatment of locally advanced or metastatic non-small cell lung cancer.Health Technol Assess. 2010 Oct;14(Suppl. 2):71-9. doi: 10.3310/hta14suppl2/10. Health Technol Assess. 2010. PMID: 21047494 Review.
-
Pemetrexed for the maintenance treatment of locally advanced or metastatic non-small cell lung cancer.Health Technol Assess. 2010 Oct;14(Suppl. 2):33-9. doi: 10.3310/hta14suppl2/05. Health Technol Assess. 2010. PMID: 21047489 Review.
Cited by
-
Cost-effectiveness analysis comparing companion diagnostic tests for EGFR, ALK, and ROS1 versus next-generation sequencing (NGS) in advanced adenocarcinoma lung cancer patients.BMC Cancer. 2020 Sep 14;20(1):875. doi: 10.1186/s12885-020-07240-2. BMC Cancer. 2020. PMID: 32928143 Free PMC article.
-
Cost-Effectiveness of Osimertinib in Treating Newly Diagnosed, Advanced EGFR-Mutation-Positive Non-Small Cell Lung Cancer.Oncologist. 2019 Mar;24(3):349-357. doi: 10.1634/theoncologist.2018-0150. Epub 2018 Sep 26. Oncologist. 2019. PMID: 30257889 Free PMC article.
-
Economic Considerations in the Use of Novel Targeted Therapies for Lung Cancer: Review of Current Literature.Pharmacoeconomics. 2017 Dec;35(12):1195-1209. doi: 10.1007/s40273-017-0563-8. Pharmacoeconomics. 2017. PMID: 28861770 Review.
-
Economic Evaluations of Pharmacogenetic and Pharmacogenomic Screening Tests: A Systematic Review. Second Update of the Literature.PLoS One. 2016 Jan 11;11(1):e0146262. doi: 10.1371/journal.pone.0146262. eCollection 2016. PLoS One. 2016. PMID: 26752539 Free PMC article.
-
Cost-effectiveness of precision diagnostic testing for precision medicine approaches against non-small-cell lung cancer: A systematic review.Mol Oncol. 2021 Oct;15(10):2672-2687. doi: 10.1002/1878-0261.13038. Epub 2021 Jul 19. Mol Oncol. 2021. PMID: 34110679 Free PMC article.
References
-
- National Cancer Institute. Costs of cancer care. http://progressreport.cancer.gov/doc_detail.asp?pid=1&did=2007&chid=75&c...
-
- National Cancer Institute. SEER cancer statistics review 1975-2008. http://seer.cancer.gov/csr/1975_2008/index.html.
-
- National Cancer Institute. SEER fast stats. http://seer.cancer.gov/faststats/selections.php?#Output.
-
- Douillard JY, Shepherd FA, Hirsh V, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non–small-cell lung cancer: Data from the randomized phase III INTEREST trial. J Clin Oncol. 2010;28:744–752. - PubMed
-
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

