Early QT assessment--how can our confidence in the data be improved?
- PMID: 23278510
- PMCID: PMC3853524
- DOI: 10.1111/bcp.12068
Early QT assessment--how can our confidence in the data be improved?
Abstract
Exposure-response (ER) analysis has emerged as an important tool to interpret QT data from thorough QT (TQT) studies and allows the prediction of effects in the targeted patient population. Recently, ER analysis has also been applied to data from early clinical pharmacology studies, such as single and multiple ascending dose studies, in which high plasma concentrations are often achieved. In line with this, there is an on-going discussion between sponsors, academicians and regulators on whether 'early QT assessment' can provide sufficiently high confidence in assessment of QT prolongation to replace the TQT study. In this article, we discuss how QT assessment can be applied to early clinical studies ('early QT assessment') and what we believe is needed to achieve the same high confidence in the data as we currently obtain from data from the TQT study. The power to exclude a QTc effect exceeding 10 ms in small sample sizes using ER analysis will be discussed and compared with time-matched analysis, as described in the ICH E14 guidance. Two examples of early QT assessment are shared; one negative and one positive, and the challenge in terms of demonstrating assay sensitivity in the absence of a pharmacological positive control will be discussed. Finally, we describe a recent research proposal, which may generate data to support the replacement of the TQT study with data from QT assessment in early phase 1 studies.
Keywords: Early QT assessment; PK/PD thorough QT study; QT; QT/QTc; healthy volunteers.
© 2012 The Authors. British Journal of Clinical Pharmacology © 2012 The British Pharmacological Society.
Figures
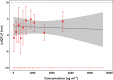
 , median concentration quantiles;
, median concentration quantiles;  , mean (90% CI) predicted QTcF prolongation
, mean (90% CI) predicted QTcF prolongation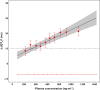
 , observed QTcF (mean ± 90% CI);
, observed QTcF (mean ± 90% CI);  , model predicted effect on QTcF (mean ± 90% CI)
, model predicted effect on QTcF (mean ± 90% CI)References
-
- ICH Harmonized Tripartite Guideline E14. The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. 2005. Available at: http://www.ich.org/cache/compo/276-254-1.html (last accessed 21 December 2012) - PubMed
-
- Shah RR. Drugs, QT interval prolongation and ICH E14: the need to get it right. Drug Saf. 2005;28:115–125. - PubMed
-
- Rodriguez I, Erdman A, Padhi D, Garnett CE, Zhao H, Targum SL, Balakrishnan S, Strnadova C, Viner N, Geiger MJ, Newton-Cheh C, Litwin J, Pugsley MK, Sager PT, Krucoff MW, Finkle JK. Electrocardiographic assessment for therapeutic proteins – scientific discussion. Am Heart J. 2010;160:627–634. - PubMed
-
- Rock EP, Finkle J, Fingert HJ, Booth BP, Garnett CE, Grant S, Justice RL, Kovacs RJ, Kowey PR, Rodriguez I, Sanhai WR, Strnadova C, Targum SL, Tsong Y, Uhl K, Stockbridge N. Assessing proarrhythmic potential of drugs when optimal studies are infeasible. Am Heart J. 2009;157:827–836. 836. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

