CD2-mediated regulation of peripheral CD4(+) CD25(+) regulatory T-cell apoptosis accompanied by down-regulation of Bim
- PMID: 23278598
- PMCID: PMC3634538
- DOI: 10.1111/imm.12054
CD2-mediated regulation of peripheral CD4(+) CD25(+) regulatory T-cell apoptosis accompanied by down-regulation of Bim
Abstract
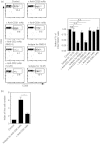
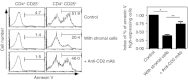
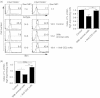
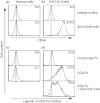
Extensive studies on CD4(+) CD25(+) regulatory T (Treg) cells suggest that they are important in regulating immune responses. However, mechanisms of peripheral Treg cell homeostasis are unknown. We found that stromal cells isolated from secondary lymphoid organs such as spleen and lymph nodes could support the survival of Treg cells. This was dependent on CD2 engagement and a direct interaction between Treg cells and stromal cells. In the presence of stromal cells, Bim, a pro-apoptotic factor, was partially decreased in Treg cells. This effect could be inhibited by anti-CD2 blocking antibodies, indicating that stimulation through CD2 on Treg cells regulates Bim expression, which may be relevant to Treg cell apoptosis. Therefore, Treg cell interactions with stromal cells through CD2 may be essential for Treg cell survival. Surprisingly, the expression of CD2 ligands on stromal cells was not detected. Hence, it is not clear how CD2 on Treg cells contributes to a direct interaction with the stromal cells and participates in survival support for Treg cells. Taken together, CD2 stimuli were mandatory for Treg cell survival with reduced Bim expression, but CD2 may not function as a direct receptor for molecules on stromal cells.
© 2012 Blackwell Publishing Ltd.
Figures



References
-
- Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25): breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. - PubMed
-
- Hori S, Takahashi T, Sakaguchi S. Control of autoimmunity by naturally arising regulatory CD4+ T cells. Adv Immunol. 2003;81:331–71. - PubMed
-
- Suvas S, Azkur AK, Kim BS, et al. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J Immunol. 2004;172:4123–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

