How the immune system talks to itself: the varied role of synapses
- PMID: 23278741
- PMCID: PMC3645447
- DOI: 10.1111/imr.12017
How the immune system talks to itself: the varied role of synapses
Abstract
Using an elaborately evolved language of cytokines and chemokines as well as cell-cell interactions, the different components of the immune system communicate with each other and orchestrate a response (or wind one down). Immunological synapses are a key feature of the system in the ways in which they can facilitate and direct these responses. Studies analyzing the structure of an immune synapse as it forms between two cells have provided insight into how the stability and kinetics of this interaction ultimately affect the sensitivity, potency, and magnitude of a given response. Furthermore, we have gained an appreciation of how the immunological synapse provides directionality and contextual cues for downstream signaling and cellular decision-making. In this review, we discuss how using a variety of techniques, developed over the last decade, have allowed us to visualize and quantify key aspects of the dynamic synaptic interface and have furthered our understanding of their function. We describe some of the many characteristics of the immunological synapse that make it a vital part of intercellular communication and some of the questions that remain to be answered.
© 2012 John Wiley & Sons A/S. Published by Blackwell Publishing Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

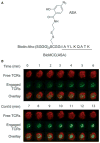


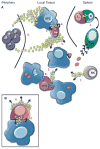
References
-
- Schluter SF, Bernstein RM, Bernstein H, Marchalonis JJ. ‘Big Bang’ emergence of the combinatorial immune system. Dev Comp Immunol. 1999;23:107–111. - PubMed
-
- Harwood NE, Batista FD. Early events in B cell activation. Annu Rev Immunol. 2010;28:185–210. - PubMed
-
- Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

