Stromal and hematopoietic cells in secondary lymphoid organs: partners in immunity
- PMID: 23278748
- PMCID: PMC3539229
- DOI: 10.1111/imr.12023
Stromal and hematopoietic cells in secondary lymphoid organs: partners in immunity
Abstract
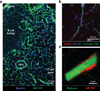
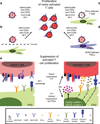
Secondary lymphoid organs (SLOs), including lymph nodes, Peyer's patches, and the spleen, have evolved to bring cells of the immune system together. In these collaborative environments, lymphocytes scan the surfaces of antigen-presenting cells for cognate antigens, while moving along stromal networks. The cell-cell interactions between stromal and hematopoietic cells in SLOs are therefore integral to the normal functioning of these tissues. Not only do stromal cells physically construct SLO architecture but they are essential for regulating hematopoietic populations within these domains. Stromal cells interact closely with lymphocytes and dendritic cells, providing scaffolds on which these cells migrate, and recruiting them into niches by secreting chemokines. Within lymph nodes, stromal cell-ensheathed conduit networks transport small antigens deep into the SLO parenchyma. More recently, stromal cells have been found to induce peripheral CD8(+) T-cell tolerance and control the extent to which newly activated T cells proliferate within lymph nodes. Thus, stromal-hematopoietic crosstalk has important consequences for regulating immune cell function within SLOs. In addition, stromal cell interactions with hematopoietic cells, other stroma, and the inflammatory milieu have profound effects on key stromal functions. Here, we examine ways in which these interactions within the lymph node environment influence the adaptive immune response.
© 2012 John Wiley & Sons A/S. Published by Blackwell Publishing Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

References
-
- Link A, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–1265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

