Cell entry of Lassa virus induces tyrosine phosphorylation of dystroglycan
- PMID: 23279385
- PMCID: PMC3805106
- DOI: 10.1111/cmi.12078
Cell entry of Lassa virus induces tyrosine phosphorylation of dystroglycan
Abstract
The extracellular matrix (ECM) receptor dystroglycan (DG) serves as a cellular receptor for the highly pathogenic arenavirus Lassa virus (LASV) that causes a haemorrhagic fever with high mortality in human. In the host cell, DG provides a molecular link between the ECM and the actin cytoskeleton via the adapter proteins utrophin or dystrophin. Here we investigated post-translational modifications of DG in the context of LASV cell entry. Using the tyrosine kinase inhibitor genistein, we found that tyrosine kinases are required for efficient internalization of virus particles, but not virus-receptor binding. Engagement of cellular DG by LASV envelope glycoprotein (LASV GP) in human epithelial cells induced tyrosine phosphorylation of the cytoplasmic domain of DG. LASV GP binding to DG further resulted in dissociation of the adapter protein utrophin from virus-bound DG. This virus-induced dissociation of utrophin was affected by genistein treatment, suggesting a role of receptor tyrosine phosphorylation in the process.
© 2012 Blackwell Publishing Ltd.
Figures


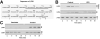

References
-
- Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119:199–207. - PubMed
-
- Belkin AM, Smalheiser NR. Localization of cranin (dystroglycan) at sites of cell-matrix and cell-cell contact: recruitment to focal adhesions is dependent upon extracellular ligands. Cell Adhes Commun. 1996;4:281–296. - PubMed
-
- Borrow P, Oldstone MB. Mechanism of lymphocytic choriomeningitis virus entry into cells. Virology. 1994;198:1–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

