Human T-cell leukemia virus type 1 Tax protein interacts with and mislocalizes the PDZ domain protein MAGI-1
- PMID: 23279616
- PMCID: PMC7657234
- DOI: 10.1111/cas.12087
Human T-cell leukemia virus type 1 Tax protein interacts with and mislocalizes the PDZ domain protein MAGI-1
Abstract
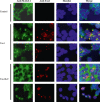
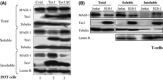
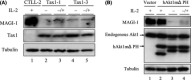
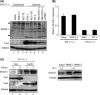
Human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent of adult T-cell leukemia (ATL). HTLV-1 encodes the oncoprotein Tax1, which is essential for immortalization of human T-cells and persistent HTLV-1 infection in vivo. Tax1 has a PDZ binding motif (PBM) at its C-terminus. This motif is crucial for the transforming activity of Tax1 to a T-cell line and persistent HTLV-1 infection. Tax1 through the PBM interacts with PDZ domain proteins such as Dlg1 and Scribble, but it has not been determined yet, which cellular PDZ proteins mediate the functions of Tax1 PBM. Here we demonstrate that Tax1 interacts with the PDZ domain protein MAGI-1 in a PBM-dependent manner, and the interaction mislocalizes MAGI-1 from the detergent-soluble to the detergent-insoluble cellular fraction in 293T cells and in HTLV-1-infected T-cells. In addition, Tax1-transformation of a T-cell line from interleukin (IL)-2-dependent to IL-2-independent growth selects cells with irreversibly reduced expression of MAGI-1 at mRNA level. These findings imply that Tax1, like other viral oncoproteins, targets MAGI-1 as a mechanism to suppress its anti-tumor functions in HTLV-1-infected cells to contribute to the transforming activity of T-cells and persistent HTLV-1 infection.
© 2012 Japanese Cancer Association.
Figures


Similar articles
-
Human T-cell leukemia virus type 1 Tax induces an aberrant clustering of the tumor suppressor Scribble through the PDZ domain-binding motif dependent and independent interaction.Virus Genes. 2008 Oct;37(2):231-40. doi: 10.1007/s11262-008-0259-4. Epub 2008 Jul 26. Virus Genes. 2008. PMID: 18661220
-
Human T-cell leukemia virus type 1 Tax oncoprotein induces and interacts with a multi-PDZ domain protein, MAGI-3.Virology. 2004 Mar 1;320(1):52-62. doi: 10.1016/j.virol.2003.11.014. Virology. 2004. PMID: 15003862
-
The PDZ domain binding motif (PBM) of human T-cell leukemia virus type 1 Tax can be substituted by heterologous PBMs from viral oncoproteins during T-cell transformation.Virus Genes. 2010 Apr;40(2):193-9. doi: 10.1007/s11262-009-0447-x. Virus Genes. 2010. PMID: 20069350
-
Distinct functions of HTLV-1 Tax1 from HTLV-2 Tax2 contribute key roles to viral pathogenesis.Retrovirology. 2009 Dec 17;6:117. doi: 10.1186/1742-4690-6-117. Retrovirology. 2009. PMID: 20017952 Free PMC article. Review.
-
The HTLV-1 Tax interactome.Retrovirology. 2008 Aug 14;5:76. doi: 10.1186/1742-4690-5-76. Retrovirology. 2008. PMID: 18702816 Free PMC article. Review.
Cited by
-
Viral PDZ Binding Motifs Influence Cell Behavior Through the Interaction with Cellular Proteins Containing PDZ Domains.Methods Mol Biol. 2021;2256:217-236. doi: 10.1007/978-1-0716-1166-1_13. Methods Mol Biol. 2021. PMID: 34014525 Review.
-
Structural insight into the Scribble PDZ domains interaction with the oncogenic Human T-cell lymphotrophic virus-1 (HTLV-1) Tax1 PBM.FEBS J. 2023 Feb;290(4):974-987. doi: 10.1111/febs.16607. Epub 2022 Sep 6. FEBS J. 2023. PMID: 36029163 Free PMC article.
-
Akt Pathway Activation by Human T-cell Leukemia Virus Type 1 Tax Oncoprotein.J Biol Chem. 2015 Oct 23;290(43):26270-81. doi: 10.1074/jbc.M115.684746. Epub 2015 Aug 31. J Biol Chem. 2015. PMID: 26324707 Free PMC article.
-
Acetylation at lysine 346 controls the transforming activity of the HTLV-1 Tax oncoprotein in the Rat-1 fibroblast model.Retrovirology. 2013 Jul 23;10:75. doi: 10.1186/1742-4690-10-75. Retrovirology. 2013. PMID: 23880157 Free PMC article.
-
Highlights on distinctive structural and functional properties of HTLV Tax proteins.Front Microbiol. 2013 Sep 9;4:271. doi: 10.3389/fmicb.2013.00271. Front Microbiol. 2013. PMID: 24058363 Free PMC article. Review.
References
-
- Yao J, Wigdahl B. Human T cell lymphotropic virus type I genomic expression and impact on intracellular signaling pathways during neurodegenerative disease and leukemia. Front Biosci 2000; 5: D138–68. - PubMed
-
- Matsuoka M, Jeang KT. Human T‐cell leukaemia virus type 1 (HTLV‐1) infectivity and cellular transformation. Nat Rev Cancer 2007; 7: 270–80. - PubMed
-
- Grassmann R, Aboud M, Jeang KT. Molecular mechanisms of cellular transformation by HTLV‐1 Tax. Oncogene 2005; 24: 5976–85. - PubMed
-
- Nerenberg M, Hinrichs SH, Reynolds RK, Khoury G, Jay G. The tat gene of human T‐lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science 1987; 237: 1324–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

