Influenza neuraminidase inhibitors: antiviral action and mechanisms of resistance
- PMID: 23279894
- PMCID: PMC4942987
- DOI: 10.1111/irv.12047
Influenza neuraminidase inhibitors: antiviral action and mechanisms of resistance
Abstract
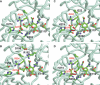
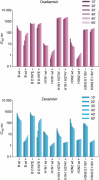
There are two major classes of antivirals available for the treatment and prevention of influenza, the M2 inhibitors and the neuraminidase inhibitors (NAIs). The M2 inhibitors are cheap, but they are only effective against influenza A viruses, and resistance arises rapidly. The current influenza A H3N2 and pandemic A(H1N1)pdm09 viruses are already resistant to the M2 inhibitors as are many H5N1 viruses. There are four NAIs licensed in some parts of the world, zanamivir, oseltamivir, peramivir, and a long-acting NAI, laninamivir. This review focuses on resistance to the NAIs. Because of differences in their chemistry and subtle differences in NA structures, resistance can be both NAI- and subtype specific. This results in different drug resistance profiles, for example, the H274Y mutation confers resistance to oseltamivir and peramivir, but not to zanamivir, and only in N1 NAs. Mutations at E119, D198, I222, R292, and N294 can also reduce NAI sensitivity. In the winter of 2007-2008, an oseltamivir-resistant seasonal influenza A(H1N1) strain with an H274Y mutation emerged in the northern hemisphere and spread rapidly around the world. In contrast to earlier evidence of such resistant viruses being unfit, this mutant virus remained fully transmissible and pathogenic and became the major seasonal A(H1N1) virus globally within a year. This resistant A(H1N1) virus was displaced by the sensitive A(H1N1)pdm09 virus. Approximately 0.5-1.0% of community A(H1N1)pdm09 isolates are currently resistant to oseltamivir. It is now apparent that variation in non-active site amino acids can affect the fitness of the enzyme and compensate for mutations that confer high-level oseltamivir resistance resulting in minimal impact on enzyme function.
© 2012 Blackwell Publishing Ltd.
Conflict of interest statement
Dr McKimm‐Breschkin has received honoraria and/or travel assistance from GlaxoSmithKline (GSK) and Hoffman La‐Roche for the participation in advisory groups and scientific meetings. She has also received research support from GSK and Biota for studies on resistance to the neuraminidase inhibitors.
Figures
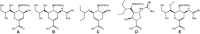
References
-
- Davies WL, Grunert RR, Haff RF et al. Antiviral Activity of 1‐Adamantanamine (Amantadine). Science 1964; 144:862–863. - PubMed
-
- Monto AS. Antivirals and influenza: frequency of resistance. Pediatr Infect Dis J 2008; 27:S110–S112. - PubMed
-
- Gubareva LV, Trujillo AA, Okomo‐Adhiambo M et al. Comprehensive assessment of 2009 pandemic influenza A (H1N1) virus drug susceptibility in vitro . Antivir Ther 2010; 15:1151–1159. - PubMed
-
- Saito R, Suzuki Y, Li D et al. Increased incidence of adamantane‐resistant influenza A(H1N1) and A(H3N2) viruses during the 2006‐2007 influenza season in Japan. J Infect Dis 2008; 197:630–632; author reply 632‐633 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

