Preferential secretion of collagen type 3 versus type 1 from adventitial fibroblasts stimulated by TGF-β/Smad3-treated medial smooth muscle cells
- PMID: 23280188
- PMCID: PMC3595331
- DOI: 10.1016/j.cellsig.2012.12.021
Preferential secretion of collagen type 3 versus type 1 from adventitial fibroblasts stimulated by TGF-β/Smad3-treated medial smooth muscle cells
Abstract
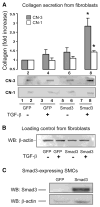
Restenosis, or arterial lumen re-narrowing, occurs in 30-50% of the patients undergoing angioplasty. Adaptive remodeling is the compensatory enlargement of the vessel size, and has been reported to prevent the deleterious effects of restenosis. Our previous studies have shown that elevated transforming growth factor (TGF-β) and its signaling protein Smad3 in the media layer induce adaptive remodeling of angioplastied rat carotid artery accompanying an increase of total collagen in the adventitia. In order to gain insights into a possible role of collagen in Smad3-induced adaptive remodeling, here we have investigated a mechanism of cell-cell communication between medial smooth muscle cells (SMCs) and adventitial fibroblasts in regulating the secretion of two major collagen subtypes. We have identified a preferential collagen-3 versus collagen-1 secretion by adventitial fibroblasts following stimulation by the conditioned medium from the TGF-β1-treated/Smad3-expressing medial smooth muscle cells (SMCs), which contained higher levels of CTGF and IGF2 as compared to control medium. Treating the TGF-β/Smad3-stimulated SMCs with an siRNA to either CTGF or IGF2 reversed the effect of conditioned media on preferential collagen-3 secretion from fibroblasts. Moreover, recombinant CTGF and IGF2 together stimulated adventitial fibroblasts to preferentially secrete collagen-3 versus collagen-1. This is the first study to identify a preferential secretion of collagen-3 versus collagen-1 from adventitial fibroblasts as a result of TGF-β/Smad3 stimulation of medial SMCs, and that CTGF and IGF2 function together to mediate this signaling communication between the two cell types.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Arterial gene transfer of the TGF-beta signalling protein Smad3 induces adaptive remodelling following angioplasty: a role for CTGF.Cardiovasc Res. 2009 Nov 1;84(2):326-35. doi: 10.1093/cvr/cvp220. Epub 2009 Jul 1. Cardiovasc Res. 2009. PMID: 19570811 Free PMC article.
-
Simvastatin inhibits transforming growth factor-β1-induced expression of type I collagen, CTGF, and α-SMA in keloid fibroblasts.Wound Repair Regen. 2014 Jan-Feb;22(1):125-33. doi: 10.1111/wrr.12136. Epub 2013 Dec 13. Wound Repair Regen. 2014. PMID: 24471776
-
Smad2 and Smad3 as mediators of the response of adventitial fibroblasts induced by transforming growth factor β1.Mol Med Rep. 2011 May-Jun;4(3):561-7. doi: 10.3892/mmr.2011.458. Epub 2011 Mar 18. Mol Med Rep. 2011. PMID: 21468608
-
Induction of renal fibrotic genes by TGF-β1 requires EGFR activation, p53 and reactive oxygen species.Cell Signal. 2013 Nov;25(11):2198-209. doi: 10.1016/j.cellsig.2013.07.007. Epub 2013 Jul 18. Cell Signal. 2013. PMID: 23872073
-
Adventitial Fibroblasts in Aortic Aneurysm: Unraveling Pathogenic Contributions to Vascular Disease.Diagnostics (Basel). 2022 Mar 31;12(4):871. doi: 10.3390/diagnostics12040871. Diagnostics (Basel). 2022. PMID: 35453919 Free PMC article. Review.
Cited by
-
Pathophysiology of thoracic aortic aneurysm (TAA): is it not one uniform aorta? Role of embryologic origin.Prog Cardiovasc Dis. 2013 Jul-Aug;56(1):68-73. doi: 10.1016/j.pcad.2013.04.002. Epub 2013 May 15. Prog Cardiovasc Dis. 2013. PMID: 23993239 Free PMC article. Review.
-
Effect of Regulatory T Cells on Promoting Apoptosis of T Lymphocyte and Its Regulatory Mechanism in Sepsis.J Interferon Cytokine Res. 2015 Dec;35(12):969-80. doi: 10.1089/jir.2014.0235. Epub 2015 Aug 26. J Interferon Cytokine Res. 2015. PMID: 26309018 Free PMC article.
-
Uighur medicine abnormal savda munzip (ASMq) suppresses expression of collagen and TGF-β1 with concomitant induce Smad7 in human hypertrophic scar fibroblasts.Int J Clin Exp Med. 2015 Jun 15;8(6):8551-60. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 26309506 Free PMC article.
-
Proinflammation, profibrosis, and arterial aging.Aging Med (Milton). 2020 Mar 18;3(3):159-168. doi: 10.1002/agm2.12099. eCollection 2020 Sep. Aging Med (Milton). 2020. PMID: 33103036 Free PMC article. Review.
-
Differential Effect of Cobalt and Chromium Ions as Well as CoCr Particles on the Expression of Osteogenic Markers and Osteoblast Function.Int J Mol Sci. 2018 Oct 5;19(10):3034. doi: 10.3390/ijms19103034. Int J Mol Sci. 2018. PMID: 30301134 Free PMC article.
References
-
- Khan R, Agrotis A, Bobik A. Cardiovascular Research. 2007;74:223–234. - PubMed
-
- Kakuta T, Currier JW, Haudenschild CC, Ryan TJ, Faxon DP. Circulation. 1994;89:2809–2815. - PubMed
-
- Mintz GS, Popma JJ, Pichard AD, Kent KM, Salter LF, Chuang YC, Griffin J, Leon MB. Journal of the American College of Cardiology. 1996;27:1678–1687. - PubMed
-
- Kingston PA, Sinha S, Appleby CE, David A, Verakis T, Castro MG, Lowenstein PR, Heagerty AM. Circulation. 2003;108:2819–2825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

