Association between adherence to National Comprehensive Cancer Network treatment guidelines and improved survival in patients with colon cancer
- PMID: 23280510
- PMCID: PMC3889139
- DOI: 10.1002/cncr.27935
Association between adherence to National Comprehensive Cancer Network treatment guidelines and improved survival in patients with colon cancer
Abstract
Background: The objective of the current study was to examine the impact of adherence to guidelines on stage-specific survival outcomes in patients with stage III and high-risk stage II colon cancer. The National Comprehensive Cancer Network (NCCN) has established working, expert consensus, and evidence-based guidelines for organ-specific cancer care, including care of patients with colon cancer.
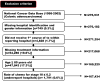
Methods: Patients who were diagnosed with colon adenocarcinoma between 1998 and 2002 were selected from within the National Cancer Data Base. The cohort was limited to patients who received their first course of treatment at the reporting facility. Pathologic variables, including tumor depth, lymph node status, and evidence of metastatic disease, were used to restage patients, and the patients were divided into low-risk and high-risk categories on the basis of criteria defined by the NCCN. Relative survival rates were calculated for the entire cohort, stratified according to adherence versus nonadherence to NCCN treatment guidelines.
Results: In univariate analysis of treatment adherence patterns for both patient subgroups (high-risk stage II and stage III), several factors were associated with a higher rate of nonadherence in both groups, including older age (P < .001); Medicaid, Medicare, or uninsured status versus private insurance (P < .001); and subsequent treatment at a facility other than the facility at which the cancer was first diagnosed (P < .001). In multivariate analysis, multiple factors were associated with differences in relative survival, although analyses that included the year of diagnosis did not demonstrate significant differences over time.
Conclusions: The current study documented practice patterns in a heterogeneous population of patients with colon cancer and demonstrated a survival benefit for patients with stage III and high-risk stage II colon cancer who received treatment that adhered to NCCN guidelines. These data validate the current NCCN practice guidelines for colon cancer and support the concept of guideline-based metrics that can be compared across institutions to assess the quality of cancer care and to compare the quality of cancer care among institutions.
Copyright © 2012 American Cancer Society.
Figures




References
-
- National Comprehensive Cancer Center N. NCCN Clinical Practice Guidelines in Oncology: Colon Cancer. [online] http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. - PubMed
-
- Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. J Clin Oncol. 1999;17(5):1356–1363. - PubMed
-
- Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27(19):3109–3116. - PubMed
-
- Laurie JA, Moertel CG, Fleming TR, et al. Surgical adjuvant therapy of large-bowel carcinoma: an evaluation of levamisole and the combination of levamisole and fluorouracil. The North Central Cancer Treatment Group and the Mayo Clinic. J Clin Oncol. 1989 Oct;7(10):1447–1456. - PubMed
-
- Moertel CG, Fleming TR, Macdonald JS, et al. Intergroup study of fluorouracil plus levamisole as adjuvant therapy for stage II/Dukes' B2 colon cancer. J Clin Oncol. 1995 Dec;13(12):2936–2943. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

