Protein kinase Cβ is required for lupus development in Sle mice
- PMID: 23280626
- PMCID: PMC3762702
- DOI: 10.1002/art.37825
Protein kinase Cβ is required for lupus development in Sle mice
Abstract
Objective: To evaluate the requirement for protein kinase Cβ (PKCβ) in the development of lupus in mice, and to explore the potential of targeting PKCβ as a therapeutic strategy in lupus.
Methods: Congenic mice bearing the disease loci Sle1 or Sle1 and Sle3, which represent different stages of severity in the development of lupus, were crossed with PKCβ-deficient mice. The effect of PKCβ deficiency in lupus development was analyzed. In addition, the effects of the PKCβ-specific inhibitor enzastaurin on the survival of B cells from mice with lupus and human 9G4-positive B cells as well as the in vivo effect of enzastaurin treatment on the development of lupus in Sle mice were investigated.
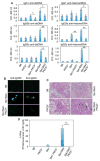
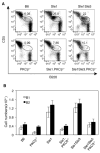
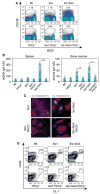
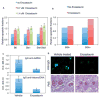
Results: In Sle mice, PKCβ deficiency abrogated lupus-associated phenotypes, including high autoantibody levels, proteinuria, and histologic features of lupus nephritis. Significant decreases in spleen size and in the peritoneal B-1 cell population, reduced numbers of activated CD4 T cells, and normalized CD4:CD8 ratios were observed. PKCβ deficiency induced an anergic B cell phenotype and preferentially inhibited autoreactive plasma cells and autoantibodies in mice with lupus. Inhibition of PKCβ enhanced apoptosis of both B cells from Sle mice and human autoreactive B cells (9G4 positive). Treatment of Sle mice with the PKCβ-specific inhibitor enzastaurin prevented the development of lupus.
Conclusion: This study identifies PKCβ as a central mediator of lupus pathogenesis, suggesting that PKCβ represents a promising therapeutic target for the treatment of systemic lupus erythematosus. Moreover, the results indicate the feasibility of using a PKCβ inhibitor for the treatment of lupus.
Copyright © 2013 by the American College of Rheumatology.
Figures


References
-
- Doreau A, Belot A, Bastid J, Riche B, Trescol-Biemont MC, Ranchin B, et al. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

