N-methyl-D-aspartate receptor antibodies in herpes simplex encephalitis
- PMID: 23280840
- PMCID: PMC3725636
- DOI: 10.1002/ana.23689
N-methyl-D-aspartate receptor antibodies in herpes simplex encephalitis
Abstract
Objective: To determine the presence and kinetics of antibodies against synaptic proteins in patients with herpes simplex virus encephalitis (HSE).
Methods: Retrospective analysis of 44 patients with polymerase chain reaction-proven HSE for the presence of a large panel of onconeuronal and synaptic receptor antibodies. The effect of patients' serum was studied in cultures of primary mouse hippocampal neurons.
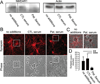
Results: N-Methyl-D-aspartate receptor (NMDAR) antibodies of the immunoglobulin (Ig) subtypes IgA, IgG, or IgM were detected in 13 of 44 patients (30%) in the course of HSE, suggesting secondary autoimmune mechanisms. NMDAR antibodies were often present at hospital admission, but in some patients developed after the first week of HSE. Antibody-positive sera resulted in downregulation of synaptic marker proteins in hippocampal neurons.
Interpretation: Some patients with HSE develop IgA, IgG, or IgM autoantibodies against NMDAR. Sera from these patients alter the density of neuronal synaptic markers, suggesting a potential pathogenic disease-modifying effect. These findings have implications for the understanding of autoimmunity in infectious diseases, and prospective studies should reveal whether the subgroup of patients with HSE and NMDAR antibodies may benefit from immunotherapy. .
Copyright © 2012 American Neurological Association.
Conflict of interest statement
C.K.: employment, Euroimmun. C.P.: employment, stock/ stock options, Euroimmun. K.B.: employment, Euroimmun. L.K.: employment, Euroimmun. W.S.: board membership, employment, stock/stock options, Euroimmun. J.D.: grants/grants pending, Euroimmun, NIH/ NCI; patents, Athena Diagnostics, Euroimmun. K.-P.W.: employment, stock/stock options, Euroimmun
Figures



References
-
- Steiner I, Kennedy PG, Pachner AR. The neurotropic herpes viruses: herpes simplex and varicella-zoster. Lancet Neurol. 2007;6:1015–1028. - PubMed
-
- Whitley R. Herpes simplex virus. In: Sheld W, Whitley R, C Marra, editors. Infections of the central nervous system. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. pp. 123–144.
-
- Raschilas F, Wolff M, Delatour F, et al. Outcome of and prognostic factors for herpes simplex encephalitis in adult patients: results of a multicenter study. Clin Infect Dis. 2002;35:254–260. - PubMed
-
- Sellner J, Dvorak F, Zhou Y, et al. Acute and long-term alteration of chemokine mRNA expression after anti-viral and anti-inflammatory treatment in herpes simplex virus encephalitis. Neurosci Lett. 2005;374:197–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

