Central axons preparing to myelinate are highly sensitive [corrected] to ischemic injury
- PMID: 23280842
- PMCID: PMC3539243
- DOI: 10.1002/ana.23690
Central axons preparing to myelinate are highly sensitive [corrected] to ischemic injury
Erratum in
- Ann Neurol. 2014 Mar;75(3):465
Abstract
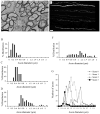
Objective: Developing central white matter is subject to ischemic-type injury during the period that precedes myelination. At this stage in maturation, central axons initiate a program of radial expansion and ion channel redistribution. Here we test the hypothesis that during radial expansion axons display heightened ischemic sensitivity, when clusters of Ca(2+) channels decorate future node of Ranvier sites.
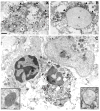
Methods: Functionality and morphology of central axons and glia were examined during and after a period of modeled ischemia. Pathological changes in axons undergoing radial expansion were probed using electrophysiological, quantitative ultrastructural, and morphometric analysis in neonatal rodent optic nerve and periventricular white matter axons studied under modeled ischemia in vitro or after hypoxia-ischemia in vivo.
Results: Acute ischemic injury of central axons undergoing initial radial expansion was mediated by Ca(2+) influx through Ca(2+) channels expressed in axolemma clusters. This form of injury operated only in this axon population, which was more sensitive to injury than neighboring myelinated axons, smaller axons yet to initiate radial expansion, astrocytes, or oligodendroglia. A pharmacological strategy designed to protect both small and large diameter premyelinated axons proved 100% protective against acute ischemia studied under modeled ischemia in vitro or after hypoxia-ischemia in vivo.
Interpretation: Recent clinical data highlight the importance of axon pathology in developing white matter injury. The elevated susceptibility of early maturing axons to ischemic injury described here may significantly contribute to selective white matter pathology and places these axons alongside preoligodendrocytes as a potential primary target of both injury and therapeutics.
Copyright © 2012 American Neurological Association.
Figures





References
-
- Back SA. Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment Retard Dev Disabil Res Rev. 2006;12:129–140. - PubMed
-
- Back SA, Rivkees SA. Emerging concepts in periventricular white matter injury. Semin Perinatol. 2004;28:405–414. - PubMed
-
- Volpe JJ. Neurology of the newborn. Philadelphia: W.B. Saunders Company; 1995.
-
- Bax M, Tydeman C, Flodmark O. Clinical and MRI correlates of cerebral palsy: the European Cerebral Palsy Study. Jama. 2006;296:1602–1608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

