Early interneuron dysfunction in ALS: insights from a mutant sod1 zebrafish model
- PMID: 23281025
- PMCID: PMC3608830
- DOI: 10.1002/ana.23780
Early interneuron dysfunction in ALS: insights from a mutant sod1 zebrafish model
Abstract
Objective: To determine, when, how, and which neurons initiate the onset of pathophysiology in amyotrophic lateral sclerosis (ALS) using a transgenic mutant sod1 zebrafish model and identify neuroprotective drugs.
Methods: Proteinopathies such as ALS involve mutant proteins that misfold and activate the heat shock stress response (HSR). The HSR is indicative of neuronal stress, and we used a fluorescent hsp70-DsRed reporter in our transgenic zebrafish to track neuronal stress and to measure functional changes in neurons and muscle over the course of the disease.
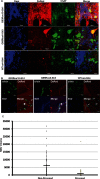
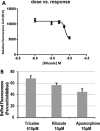
Results: We show that mutant sod1 fish first exhibited the HSR in glycinergic interneurons at 24 hours postfertilization (hpf). By 96 hpf, we observed a significant reduction in spontaneous glycinergic currents induced in spinal motor neurons. The loss of inhibition was followed by increased stress in the motor neurons of symptomatic adults and concurrent morphological changes at the neuromuscular junction (NMJ) indicative of denervation. Riluzole, the only approved ALS drug and apomorphine, an NRF2 activator, reduced the observed early neuronal stress response.
Interpretation: The earliest event in the pathophysiology of ALS in the mutant sod1 zebrafish model involves neuronal stress in inhibitory interneurons, resulting from mutant Sod1 expression. This is followed by a reduction in inhibitory input to motor neurons. The loss of inhibitory input may contribute to the later development of neuronal stress in motor neurons and concurrent inability to maintain the NMJ. Riluzole, the approved drug for use in ALS, modulates neuronal stress in interneurons, indicating a novel mechanism of riluzole action.
Copyright © 2012 American Neurological Association.
Figures




Similar articles
-
Spinal inhibitory interneuron pathology follows motor neuron degeneration independent of glial mutant superoxide dismutase 1 expression in SOD1-ALS mice.J Neuropathol Exp Neurol. 2011 Aug;70(8):662-77. doi: 10.1097/NEN.0b013e31822581ac. J Neuropathol Exp Neurol. 2011. PMID: 21760539
-
Locomotor deficits in a mouse model of ALS are paralleled by loss of V1-interneuron connections onto fast motor neurons.Nat Commun. 2021 May 31;12(1):3251. doi: 10.1038/s41467-021-23224-7. Nat Commun. 2021. PMID: 34059686 Free PMC article.
-
Neuromuscular effects of G93A-SOD1 expression in zebrafish.Mol Neurodegener. 2012 Aug 31;7:44. doi: 10.1186/1750-1326-7-44. Mol Neurodegener. 2012. PMID: 22938571 Free PMC article.
-
Transgenic mouse model for familial amyotrophic lateral sclerosis with superoxide dismutase-1 mutation.Neuropathology. 2001 Mar;21(1):82-92. doi: 10.1046/j.1440-1789.2001.00361.x. Neuropathology. 2001. PMID: 11304046 Review.
-
Neuromuscular junction destruction during amyotrophic lateral sclerosis: insights from transgenic models.Curr Opin Pharmacol. 2009 Jun;9(3):341-6. doi: 10.1016/j.coph.2009.03.007. Epub 2009 Apr 20. Curr Opin Pharmacol. 2009. PMID: 19386549 Review.
Cited by
-
Exciting Complexity: The Role of Motor Circuit Elements in ALS Pathophysiology.Front Neurosci. 2020 Jun 17;14:573. doi: 10.3389/fnins.2020.00573. eCollection 2020. Front Neurosci. 2020. PMID: 32625051 Free PMC article. Review.
-
Function Over Form: Modeling Groups of Inherited Neurological Conditions in Zebrafish.Front Mol Neurosci. 2016 Jul 7;9:55. doi: 10.3389/fnmol.2016.00055. eCollection 2016. Front Mol Neurosci. 2016. PMID: 27458342 Free PMC article. Review.
-
Zebrafish: A marvel of high-throughput biology for 21st century toxicology.Curr Environ Health Rep. 2014 Sep 7;1(4):341-352. doi: 10.1007/s40572-014-0029-5. Curr Environ Health Rep. 2014. PMID: 25678986 Free PMC article.
-
INaP selective inhibition reverts precocious inter- and motorneurons hyperexcitability in the Sod1-G93R zebrafish ALS model.Sci Rep. 2016 Apr 15;6:24515. doi: 10.1038/srep24515. Sci Rep. 2016. PMID: 27079797 Free PMC article.
-
Going the Extra (Synaptic) Mile: Excitotoxicity as the Road Toward Neurodegenerative Diseases.Front Cell Neurosci. 2020 Apr 24;14:90. doi: 10.3389/fncel.2020.00090. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32390802 Free PMC article. Review.
References
-
- Turner BJ, Talbot K. Transgenics, toxicity and therapeutics in rodent models of mutant SOD1-mediated familial ALS. Prog Neurobiol. 2008;85:94–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

