Insulin analog with additional disulfide bond has increased stability and preserved activity
- PMID: 23281053
- PMCID: PMC3595460
- DOI: 10.1002/pro.2211
Insulin analog with additional disulfide bond has increased stability and preserved activity
Abstract
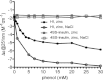
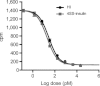
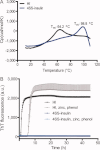
Insulin is a key hormone controlling glucose homeostasis. All known vertebrate insulin analogs have a classical structure with three 100% conserved disulfide bonds that are essential for structural stability and thus the function of insulin. It might be hypothesized that an additional disulfide bond may enhance insulin structural stability which would be highly desirable in a pharmaceutical use. To address this hypothesis, we designed insulin with an additional interchain disulfide bond in positions A10/B4 based on Cα-Cα distances, solvent exposure, and side-chain orientation in human insulin (HI) structure. This insulin analog had increased affinity for the insulin receptor and apparently augmented glucodynamic potency in a normal rat model compared with HI. Addition of the disulfide bond also resulted in a 34.6°C increase in melting temperature and prevented insulin fibril formation under high physical stress even though the C-terminus of the B-chain thought to be directly involved in fibril formation was not modified. Importantly, this analog was capable of forming hexamer upon Zn addition as typical for wild-type insulin and its crystal structure showed only minor deviations from the classical insulin structure. Furthermore, the additional disulfide bond prevented this insulin analog from adopting the R-state conformation and thus showing that the R-state conformation is not a prerequisite for binding to insulin receptor as previously suggested. In summary, this is the first example of an insulin analog featuring a fourth disulfide bond with increased structural stability and retained function.
Copyright © 2012 The Protein Society.
Figures
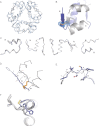

References
-
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications (1999) Part 1: Diagnosis and Classification of Diabetes Mellitus. WHO Consultation. Geneva: World Health Organization, Department of Noncommunicable Disease Surveillance;
-
- Baker EN, Blundell TL, Cutfield JF, Cutfield SM, Dodson EJ, Dodson GG, Hodgkin DM, Hubbard RE, Isaacs NW, Reynolds CD, Sakabe K, Sakabe N, Vijayan NM. The structure of 2Zn pig insulin crystals at 1.5 A resolution. Philos Trans R Soc Lond B Biol Sci. 1988;319:369–456. - PubMed
-
- Blundell T, Dodson G, Hodgkin D, Mercola D. Insulin: The structure in the crystal and its reflection in chemistry and biology. Adv Protein Chem. 1972;26:279–402.
-
- Nicol DS, Smith LF. Amino-acid sequence of human insulin. Nature. 1960;187:483–485. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

