pH-responsive hydrogels with dispersed hydrophobic nanoparticles for the oral delivery of chemotherapeutics
- PMID: 23281185
- PMCID: PMC3619027
- DOI: 10.1002/jbm.a.34532
pH-responsive hydrogels with dispersed hydrophobic nanoparticles for the oral delivery of chemotherapeutics
Abstract
Amphiphilic polymer carriers were formed by polymerizing a hydrophilic, pH-responsive hydrogel composed of poly(methacrylic-grafted-ethylene glycol) (P(MAA-g-EG)) in the presence of hydrophobic PMMA nanoparticles. These polymer carriers were varied in PMMA nanoparticle content to elicit a variety of physiochemical properties which would preferentially load doxorubicin, a hydrophobic chemotherapeutic, and release doxorubicin locally in the colon for the treatment of colon cancers. Loading levels ranged from 49% to 64% and increased with increasing nanoparticle content. Doxorubicin loaded polymers were released in a physiological model where low pH was used to simulate the stomach and then stepped to more neutral conditions to simulate the upper small intestine. P(MAA-g-EG) containing nanoparticles were less mucoadhesive as determined using a tensile tester, polymer samples, and fresh porcine small intestine. The cytocompatibility of the polymer materials were assessed using cell lines representing the GI tract and colon cancer and were noncytotoxic at varying concentrations and exposure times.
Copyright © 2012 Wiley Periodicals, Inc.
Figures

 ), P(MAA-g-EG)-2.5NP (▲), and P(MAA-g-EG)-5.0NP (○) crushed particles (75 – 150 µm) were released in 1× PBS (pH 7.4) for 6 hr. Doxorubicin release is expressed as Mt/M∞. Curves generated are n = 3 and error bars represent error propagation due to ratio of Mt/M∞. M∞ ranged was 42.5, 18.7, 29.4, and 33.7 µg/mL for P(MAA-g-EG), P(MAA-g-EG)-1.0NP, P(MAA-g-EG)-2.5NP, and P(MAA-g-EG)-5.0NP, respectively.
), P(MAA-g-EG)-2.5NP (▲), and P(MAA-g-EG)-5.0NP (○) crushed particles (75 – 150 µm) were released in 1× PBS (pH 7.4) for 6 hr. Doxorubicin release is expressed as Mt/M∞. Curves generated are n = 3 and error bars represent error propagation due to ratio of Mt/M∞. M∞ ranged was 42.5, 18.7, 29.4, and 33.7 µg/mL for P(MAA-g-EG), P(MAA-g-EG)-1.0NP, P(MAA-g-EG)-2.5NP, and P(MAA-g-EG)-5.0NP, respectively.
 ), P(MAA-g-EG)-2.5NP (▲), and P(MAA-g-EG)-5.0NP (○) crushed particles (75 – 150 µm) were released in 1× PBS (pH 2.0) for 90 min. Then the pH was increased to 7.0 by adding 5 N NaOH and release continued for 6 hr. Doxorubicin release is expressed as Mt/M∞. Curves generated are n = 3 and error bars represent error propagation due to ratio of Mt/M∞. M∞ ranged was 42.5, 18.7, 29.4, and 33.7 µg/mL for P(MAA-g-EG), P(MAA-g-EG)-1.0NP, P(MAA-g-EG)-2.5NP, and P(MAA-g-EG)-5.0NP, respectively.
), P(MAA-g-EG)-2.5NP (▲), and P(MAA-g-EG)-5.0NP (○) crushed particles (75 – 150 µm) were released in 1× PBS (pH 2.0) for 90 min. Then the pH was increased to 7.0 by adding 5 N NaOH and release continued for 6 hr. Doxorubicin release is expressed as Mt/M∞. Curves generated are n = 3 and error bars represent error propagation due to ratio of Mt/M∞. M∞ ranged was 42.5, 18.7, 29.4, and 33.7 µg/mL for P(MAA-g-EG), P(MAA-g-EG)-1.0NP, P(MAA-g-EG)-2.5NP, and P(MAA-g-EG)-5.0NP, respectively.

 ), and P(MAA-g-EG)-5.0NP (■) microparticles (75 – 150 µm) were added to all cell lines and incubated for 2 hr. Error bars represent error propagated over control cells. n = 6 – 8.
), and P(MAA-g-EG)-5.0NP (■) microparticles (75 – 150 µm) were added to all cell lines and incubated for 2 hr. Error bars represent error propagated over control cells. n = 6 – 8.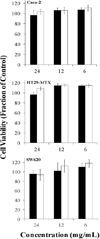

References
-
- Blanchette J, Kavimandan N, Peppas NA. Principles of transmucosal delivery of therapeutic agents. Biomed Pharmacother. 2004;58:142–151. - PubMed
-
- Liu G, Franssen E, Fitch MI, Warner E. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997;15:110–115. - PubMed
-
- Irshad S, Maisey N. Considerations when choosing oral chemotherapy: identifying and responding to patient need. Eur J Cancer Care. 2010;19:5–11.
-
- Mao JH, Balmain A. Genomic approaches to identification of tumour-susceptibility genes using mouse models. Curr Opin Genet Dev. 2003;13:14–19. - PubMed
-
- Ohmori K, Sasaki K, Asada S, Tanaka N, Umeda M. An assay method for the prediction of tumor promoting potential of chemicals by the use of Bhas 42 cells. Mutat Res-Gen Tox En. 2004;557:191–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

