Low-level laser therapy (LLLT) reduces oxidative stress in primary cortical neurons in vitro
- PMID: 23281261
- PMCID: PMC3651776
- DOI: 10.1002/jbio.201200157
Low-level laser therapy (LLLT) reduces oxidative stress in primary cortical neurons in vitro
Abstract
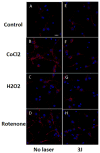
Low-level laser (light) therapy (LLLT) involves absorption of photons being in the mitochondria of cells leading to improvement in electron transport, increased mitochondrial membrane potential (MMP), and greater ATP production. Low levels of reactive oxygen species (ROS) are produced by LLLT in normal cells that are beneficial. We exposed primary cultured murine cortical neurons to oxidative stressors: hydrogen peroxide, cobalt chloride and rotenone in the presence or absence of LLLT (3 J/cm², CW, 810 nm wavelength laser, 20 mW/cm²). Cell viability was determined by Prestoblue™ assay. ROS in mitochondria was detected using Mito-sox, while ROS in cytoplasm was detected with CellRox™. MMP was measured with tetramethylrhodamine. In normal neurons LLLT elevated MMP and increased ROS. In oxidatively-stressed cells LLLT increased MMP but reduced high ROS levels and protected cultured cortical neurons from death. Although LLLT increases ROS in normal neurons, it reduces ROS in oxidatively-stressed neurons. In both cases MMP is increased. These data may explain how LLLT can reduce clinical oxidative stress in various lesions while increasing ROS in cells in vitro.
Keywords: cobalt chloride; cultured cortical neurons; hydrogen peroxide; low-level laser therapy; oxidative stress; reactive oxygen species.
Copyright © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures









References
-
- Byrnes KR, Waynant RW, Ilev IK, Wu X, Barna L, Smith K, Heckert R, Gerst H, Anders JJ. Lasers Surg Med. 2005;36:171–185. - PubMed
-
- Wu X, Dmitriev AE, Cardoso MJ, Viers-Costello AG, Borke RC, Streeter J, Anders JJ. Lasers Surg Med. 2009;41:36–41. - PubMed
-
- Rochkind S. Neurosurg Focus. 2009;26:E8. - PubMed
-
- Oron A, Oron U, Chen J, Eilam A, Zhang C, Sadeh M, Lampl Y, Streeter J, DeTaboada L, Chopp M. Stroke. 2006;37:2620–2624. - PubMed
-
- Detaboada L, Ilic S, Leichliter-Martha S, Oron U, Oron A, Streeter J. Lasers Surg Med. 2006;38:70–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

