CXCR5⁺ T helper cells mediate protective immunity against tuberculosis
- PMID: 23281399
- PMCID: PMC3561804
- DOI: 10.1172/JCI65728
CXCR5⁺ T helper cells mediate protective immunity against tuberculosis
Abstract
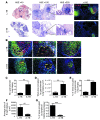
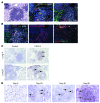
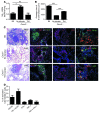
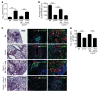
One third of the world's population is infected with Mycobacterium tuberculosis (Mtb). Although most infected people remain asymptomatic, they have a 10% lifetime risk of developing active tuberculosis (TB). Thus, the current challenge is to identify immune parameters that distinguish individuals with latent TB from those with active TB. Using human and experimental models of Mtb infection, we demonstrated that organized ectopic lymphoid structures containing CXCR5+ T cells were present in Mtb-infected lungs. In addition, we found that in experimental Mtb infection models, the presence of CXCR5+ T cells within ectopic lymphoid structures was associated with immune control. Furthermore, in a mouse model of Mtb infection, we showed that activated CD4+CXCR5+ T cells accumulated in Mtb-infected lungs and produced proinflammatory cytokines. Mice deficient in Cxcr5 had increased susceptibility to TB due to defective T cell localization within the lung parenchyma. We demonstrated that CXCR5 expression in T cells mediated correct T cell localization within TB granulomas, promoted efficient macrophage activation, protected against Mtb infection, and facilitated lymphoid follicle formation. These data demonstrate that CD4+CXCR5+ T cells play a protective role in the immune response against TB and highlight their potential use for future TB vaccine design and therapy.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- AI083541/AI/NIAID NIH HHS/United States
- AI91036/AI/NIAID NIH HHS/United States
- R01 AI060422/AI/NIAID NIH HHS/United States
- HL105427/HL/NHLBI NIH HHS/United States
- P51 RR000164/RR/NCRR NIH HHS/United States
- P51 OD011104/OD/NIH HHS/United States
- R01 HL105427/HL/NHLBI NIH HHS/United States
- RR020159/RR/NCRR NIH HHS/United States
- AI060422/AI/NIAID NIH HHS/United States
- RR000164/RR/NCRR NIH HHS/United States
- P20 RR020159/RR/NCRR NIH HHS/United States
- HL69409/HL/NHLBI NIH HHS/United States
- T32 AI065380/AI/NIAID NIH HHS/United States
- R01 HL069409/HL/NHLBI NIH HHS/United States
- R21 AI091457/AI/NIAID NIH HHS/United States
- AI091457/AI/NIAID NIH HHS/United States
- R21 AI083541/AI/NIAID NIH HHS/United States
- RR026006/RR/NCRR NIH HHS/United States
- T32 AI065380-08/AI/NIAID NIH HHS/United States
- U19 AI091036/AI/NIAID NIH HHS/United States
- R21 RR026006/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

