Signaling effectors underlying pathologic growth and remodeling of the heart
- PMID: 23281408
- PMCID: PMC3533272
- DOI: 10.1172/JCI62839
Signaling effectors underlying pathologic growth and remodeling of the heart
Abstract
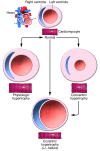
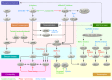
Cardiovascular disease is the number one cause of mortality in the Western world. The heart responds to many cardiopathological conditions with hypertrophic growth by enlarging individual myocytes to augment cardiac pump function and decrease ventricular wall tension. Initially, such cardiac hypertrophic growth is often compensatory, but as time progresses these changes become maladaptive. Cardiac hypertrophy is the strongest predictor for the development of heart failure, arrhythmia, and sudden death. Here we discuss therapeutic avenues emerging from molecular and genetic studies of cardiovascular disease in animal models. The majority of these are based on intracellular signaling pathways considered central to pathologic cardiac remodeling and hypertrophy, which then leads to heart failure. We focus our discussion on selected therapeutic targets that have more recently emerged and have a tangible translational potential given the available pharmacologic agents that could be readily evaluated in human clinical trials.
Figures