Hypoxia-inducible factor 1 is essential for spontaneous recovery from traumatic brain injury and is a key mediator of heat acclimation induced neuroprotection
- PMID: 23281425
- PMCID: PMC3618386
- DOI: 10.1038/jcbfm.2012.193
Hypoxia-inducible factor 1 is essential for spontaneous recovery from traumatic brain injury and is a key mediator of heat acclimation induced neuroprotection
Abstract
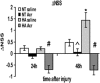
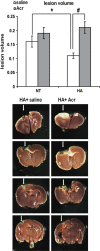
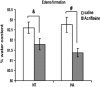
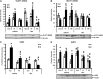
Heat acclimation (HA), a well-established preconditioning model, confers neuroprotection in rodent models of traumatic brain injury (TBI). It increases neuroprotective factors, among them is hypoxia-inducible factor 1α (HIF-1α), which is important in the response to postinjury ischemia. However, little is known about the role of HIF-1α in TBI and its contribution to the establishment of the HA protecting phenotype. Therefore, we aimed to explore HIF-1α role in TBI defense mechanisms as well as in HA-induced neuroprotection. Acriflavine was used to inhibit HIF-1 in injured normothermic (NT) or HA mice. After TBI, we evaluated motor function recovery, lesion volume, edema formation, and body temperature as well as HIF-1 downstream transcription targets, such as glucose transporter 1 (GLUT1), vascular endothelial growth factor, and aquaporin 4. We found that HIF-1 inhibition resulted in deterioration of motor function, increased lesion volume, hypothermia, and reduced edema formation. All these parameters were significantly different in the HA mice. Western blot analysis and enzyme-linked immunosorbent assay showed reduced levels of all HIF-1 downstream targets in HA mice, however, only GLUT1 was downregulated in NT mice. We conclude that HIF-1 is a key mediator in both spontaneous recovery and HA-induced neuroprotection after TBI.
Figures


References
-
- Beit-Yannai E, Kohen R, Horowitz M, Trembovler V, Shohami E. Changes of biological reducing activity in rat brain following closed head injury: a cyclic voltammetry study in normal and heat-acclimated rats. J Cereb Blood Flow Metab. 1997;17:273–279. - PubMed
-
- Shein NA, Horowitz M, Alexandrovich AG, Tsenter J, Shohami E. Heat acclimation increases hypoxia-inducible factor 1alpha and erythropoietin receptor expression: implication for neuroprotection after closed head injury in mice. J Cereb Blood Flow Metab. 2005;25:1456–1465. - PubMed
-
- Leker RR, Shohami E. Cerebral ischemia and trauma-different etiologies yet similar mechanisms: neuroprotective opportunities. Brain Res Brain Res Rev. 2002;39:55–73. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

