Plasma surface chemical treatment of electrospun poly(L-lactide) microfibrous scaffolds for enhanced cell adhesion, growth, and infiltration
- PMID: 23281641
- PMCID: PMC3609642
- DOI: 10.1089/ten.TEA.2011.0725
Plasma surface chemical treatment of electrospun poly(L-lactide) microfibrous scaffolds for enhanced cell adhesion, growth, and infiltration
Abstract
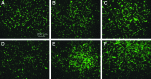
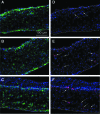
Poly(l-lactide) (PLLA) microfibrous scaffolds produced by electrospinning were treated with mild Ar or Ar-NH3/H2 plasmas to enhance cell attachment, growth, and infiltration. Goniometry, atomic force microscopy (AFM), and X-ray photoelectron spectroscopy (XPS) measurements were used to evaluate the modification of the scaffold surface chemistry by plasma treatment. AFM and XPS measurements showed that both plasma treatments increased the hydrophilicity without affecting the integrity of the fibrous structure and the fiber roughness, whereas Ar-NH3/H2 plasma treatment also resulted in surface functionalization with amine groups. Culture studies of bovine aorta endothelial cells and bovine smooth muscle cells on the plasma-treated PLLA scaffolds revealed that both Ar and Ar-NH3/H2 plasma treatments promoted cell spreading during the initial stage of cell attachment and, more importantly, increased the cell growth rate, especially for Ar plasma treatment. In vitro cell infiltration studies showed that both plasma treatments effectively enhanced cell migration into the microfibrous scaffolds. In vivo experiments involving the subcutaneous implantation of plasma-treated PLLA scaffolds under the skin of Sprague-Dawley rats also showed increased cell infiltration. The results of this study indicate that surface treatment of PLLA microfibrous scaffolds with mild Ar or Ar-NH3/H2 plasmas may have important implications in tissue engineering. Further modifications with bioactive factors should improve the functions of the scaffolds for specific applications.
Figures








Similar articles
-
Plasma-assisted heparin conjugation on electrospun poly(L-lactide) fibrous scaffolds.J Biomed Mater Res A. 2014 May;102(5):1408-14. doi: 10.1002/jbm.a.34802. Epub 2013 Jun 20. J Biomed Mater Res A. 2014. PMID: 23681664 Free PMC article.
-
Cold atmospheric plasma (CAP) surface nanomodified 3D printed polylactic acid (PLA) scaffolds for bone regeneration.Acta Biomater. 2016 Dec;46:256-265. doi: 10.1016/j.actbio.2016.09.030. Epub 2016 Sep 22. Acta Biomater. 2016. PMID: 27667017
-
Aligned PLLA nanofibrous scaffolds coated with graphene oxide for promoting neural cell growth.Acta Biomater. 2016 Jun;37:131-42. doi: 10.1016/j.actbio.2016.04.008. Epub 2016 Apr 7. Acta Biomater. 2016. PMID: 27063493
-
An improved surface for enhanced stem cell proliferation and osteogenic differentiation using electrospun composite PLLA/P123 scaffold.Artif Cells Nanomed Biotechnol. 2018 Sep;46(6):1274-1281. doi: 10.1080/21691401.2017.1367928. Epub 2017 Aug 24. Artif Cells Nanomed Biotechnol. 2018. PMID: 28835133
-
DBD atmospheric plasma-modified, electrospun, layer-by-layer polymeric scaffolds for L929 fibroblast cell cultivation.J Biomater Sci Polym Ed. 2016;27(2):111-32. doi: 10.1080/09205063.2015.1111717. Epub 2015 Nov 26. J Biomater Sci Polym Ed. 2016. PMID: 26494511
Cited by
-
Hydrophilic Surface Functionalization of Electrospun Nanofibrous Scaffolds in Tissue Engineering.Polymers (Basel). 2020 Nov 10;12(11):2636. doi: 10.3390/polym12112636. Polymers (Basel). 2020. PMID: 33182617 Free PMC article. Review.
-
Gelatin Blends Enhance Performance of Electrospun Polymeric Scaffolds in Comparison to Coating Protocols.Polymers (Basel). 2022 Mar 24;14(7):1311. doi: 10.3390/polym14071311. Polymers (Basel). 2022. PMID: 35406188 Free PMC article.
-
Biocompatibility of Cyclopropylamine-Based Plasma Polymers Deposited at Sub-Atmospheric Pressure on Poly (ε-caprolactone) Nanofiber Meshes.Nanomaterials (Basel). 2019 Aug 28;9(9):1215. doi: 10.3390/nano9091215. Nanomaterials (Basel). 2019. PMID: 31466357 Free PMC article.
-
Advanced Graft Development Approaches for ACL Reconstruction or Regeneration.Biomedicines. 2023 Feb 9;11(2):507. doi: 10.3390/biomedicines11020507. Biomedicines. 2023. PMID: 36831043 Free PMC article. Review.
-
3D-Printed Poly(ε-Caprolactone)/Hydroxyapatite Scaffolds Modified with Alkaline Hydrolysis Enhance Osteogenesis In Vitro.Polymers (Basel). 2021 Jan 14;13(2):257. doi: 10.3390/polym13020257. Polymers (Basel). 2021. PMID: 33466736 Free PMC article.
References
-
- Yoo H.S. Kim T.G. Park T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv Drug Deliv Rev. 2009;61:1033. - PubMed
-
- Gugala Z. Gogolewski S. Attachment, growth, and activity of rat osteoblasts on polylactide membranes treated with various low-temperature radiofrequency plasmas. J Biomed Mater Res. 2006;76A:288. - PubMed
-
- Latkany R. Tsuk A. Sheu M.-S. Loh I.-H. Trinkaus–Randall V. Plasma surface modification of artificial corneas for optimal epithelialization. J Biomed Mater Res. 1997;36:29. - PubMed
-
- Chim H. Ong J.L. Schantz J.-T. Hutmacher D.W. Agrawal C.M. Efficacy of glow discharge gas plasma treatment as a surface modification process for three-dimensional poly (d,l-lactide) scaffolds. J Biomed Mater Res. 2003;65A:327. - PubMed
-
- Baek H.S. Park Y.H. Ki C.S. Park J.-C. Rah D.K. Enhanced chondrogenic responses of articular chondrocytes onto porous silk fibroin scaffolds treated with microwave-induced argon plasma. Surf Coat Technol. 2008;202:5794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

