B-cell selection and the development of autoantibodies
- PMID: 23281837
- PMCID: PMC3535718
- DOI: 10.1186/ar3918
B-cell selection and the development of autoantibodies
Abstract
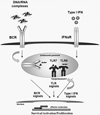
The clearest evidence that B cells play an important role in human autoimmunity is that immunotherapies that deplete B cells are very effective treatments for many autoimmune diseases. All people, healthy or ill, have autoreactive B cells, but not at the same frequency. A number of genes influence the level of these autoreactive B cells and whether they are eliminated or not during development at a central checkpoint in the bone marrow (BM) or at a later checkpoint in peripheral lymphoid tissues. These genes include those encoding proteins that regulate signaling through the B-cell receptor complex such as Btk and PTPN22, proteins that regulate innate signaling via Toll-like receptors (TLRs) such as MyD88 and interleukin-1 receptor-associated kinase 4, as well as the gene encoding the activation-induced deaminase (AID) essential for B cells to undergo class switch recombination and somatic hypermutation. Recent studies have revealed that TLR signaling elements and AID function not only in peripheral B cells to help mediate effective antibody responses to foreign antigens, but also in the BM to help remove autoreactive B-lineage cells at a very early point in B-cell development. Newly arising B cells that leave the BM and enter the blood and splenic red pulp can express both AID and TLR signaling elements like TLR7, and thus are fully equipped to respond rapidly to antigens (including autoantigens), to isotype class switch, and to undergo somatic hypermutation. These red pulp B cells may thus be an important source of autoantibody-producing cells arising particularly in extrafollicular sites, and indeed may be as significant a source of autoantibody-producing cells as B cells arising from germinal centers.
Figures


Similar articles
-
B cell proliferation, somatic hypermutation, class switch recombination, and autoantibody production in ectopic lymphoid tissue in murine lupus.J Immunol. 2009 Apr 1;182(7):4226-36. doi: 10.4049/jimmunol.0800771. J Immunol. 2009. PMID: 19299721 Free PMC article.
-
Deletion of WASp and N-WASp in B cells cripples the germinal center response and results in production of IgM autoantibodies.J Autoimmun. 2015 Aug;62:81-92. doi: 10.1016/j.jaut.2015.06.003. Epub 2015 Jul 2. J Autoimmun. 2015. PMID: 26143192 Free PMC article.
-
A new site-directed transgenic rheumatoid factor mouse model demonstrates extrafollicular class switch and plasmablast formation.Autoimmunity. 2010 Dec;43(8):607-18. doi: 10.3109/08916930903567500. Epub 2010 Apr 7. Autoimmunity. 2010. PMID: 20370572 Free PMC article.
-
Self-Reactive B Cells in the Germinal Center Reaction.Annu Rev Immunol. 2018 Apr 26;36:339-357. doi: 10.1146/annurev-immunol-051116-052510. Epub 2018 Jan 22. Annu Rev Immunol. 2018. PMID: 29356584 Review.
-
Potential roles of activation-induced cytidine deaminase in promotion or prevention of autoimmunity in humans.Autoimmunity. 2013 Mar;46(2):148-56. doi: 10.3109/08916934.2012.750299. Epub 2013 Jan 10. Autoimmunity. 2013. PMID: 23215867 Free PMC article. Review.
Cited by
-
Methotrexate Reduces the Probability of Discontinuation of TNF Inhibitors in Seropositive Patients With Rheumatoid Arthritis. A Real-World Data Analysis.Front Med (Lausanne). 2021 Jun 29;8:692557. doi: 10.3389/fmed.2021.692557. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34268325 Free PMC article.
-
Low Serum BAFF Concentration Is Associated with Response to TNF Inhibitors in Seropositive Patients with Rheumatoid Arthritis.J Clin Med. 2022 Sep 2;11(17):5207. doi: 10.3390/jcm11175207. J Clin Med. 2022. PMID: 36079136 Free PMC article.
-
Autoantibodies and B Cells: The ABC of rheumatoid arthritis pathophysiology.Immunol Rev. 2020 Mar;294(1):148-163. doi: 10.1111/imr.12829. Epub 2019 Dec 16. Immunol Rev. 2020. PMID: 31845355 Free PMC article. Review.
-
Endogenously produced catecholamines improve the regulatory function of TLR9-activated B cells.PLoS Biol. 2022 Jan 24;20(1):e3001513. doi: 10.1371/journal.pbio.3001513. eCollection 2022 Jan. PLoS Biol. 2022. PMID: 35073310 Free PMC article.
-
Sex differences in neuro(auto)immunity and chronic sciatic nerve pain.Biol Sex Differ. 2020 Nov 12;11(1):62. doi: 10.1186/s13293-020-00339-y. Biol Sex Differ. 2020. PMID: 33183347 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

