Variable expressivity of ciliopathy neurological phenotypes that encompass Meckel-Gruber syndrome and Joubert syndrome is caused by complex de-regulated ciliogenesis, Shh and Wnt signalling defects
- PMID: 23283079
- PMCID: PMC3596847
- DOI: 10.1093/hmg/dds546
Variable expressivity of ciliopathy neurological phenotypes that encompass Meckel-Gruber syndrome and Joubert syndrome is caused by complex de-regulated ciliogenesis, Shh and Wnt signalling defects
Abstract
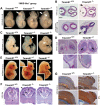
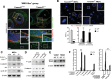
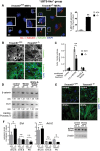
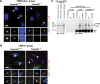
The ciliopathies are a group of heterogeneous diseases with considerable variations in phenotype for allelic conditions such as Meckel-Gruber syndrome (MKS) and Joubert syndrome (JBTS) even at the inter-individual level within families. In humans, mutations in TMEM67 (also known as MKS3) cause both MKS and JBTS, with TMEM67 encoding the orphan receptor meckelin (TMEM67) that localizes to the ciliary transition zone. We now describe the Tmem67(tm1(Dgen/H)) knockout mouse model that recapitulates the brain phenotypic variability of these human ciliopathies, with categorization of Tmem67 mutant animals into two phenotypic groups. An MKS-like incipient congenic group (F6 to F10) manifested very variable neurological features (including exencephaly, and frontal/occipital encephalocele) that were associated with the loss of primary cilia, diminished Shh signalling and dorsalization of the caudal neural tube. The 'MKS-like' group also had high de-regulated canonical Wnt/β-catenin signalling associated with hyper-activated Dishevelled-1 (Dvl-1) localized to the basal body. Conversely, a second fully congenic group (F > 10) had less variable features pathognomonic for JBTS (including cerebellar hypoplasia), and retention of abnormal bulbous cilia associated with mild neural tube ventralization. The 'JBTS-like' group had de-regulated low levels of canonical Wnt signalling associated with the loss of Dvl-1 localization to the basal body. Our results suggest that modifier alleles partially determine the variation between MKS and JBTS, implicating the interaction between Dvl-1 and meckelin, or other components of the ciliary transition zone. The Tmem67(tm1(Dgen/H)) line is unique in modelling the variable expressivity of phenotypes in these two ciliopathies.
Figures



References
-
- Alexiev B.A., Lin X., Sun C.-C., Brenner D.S. Meckel-Gruber syndrome: pathologic manifestations, minimal diagnostic criteria, and differential diagnosis. Arch. Pathol. Lab. Med. 2006;130:1236–1238. - PubMed
-
- Ahdabbarmada M., Claassen D. A distinctive triad of malformations of the central-nervous-system in the Meckel-Gruber syyndrome. J. Neuropathol. Exp. Neurol. 1990;49:610–620. - PubMed
-
- Paetau A., Salonen R., Haltia M. Brain pathology in the Meckel syndrome—a study of 59 cases. Clin. Neuropathol. 1985;4:56–62. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

