Tangential tether extraction and spontaneous tether retraction of human neutrophils
- PMID: 23283224
- PMCID: PMC3514515
- DOI: 10.1016/j.bpj.2012.10.018
Tangential tether extraction and spontaneous tether retraction of human neutrophils
Abstract
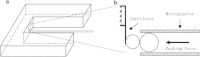
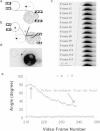
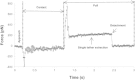
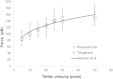
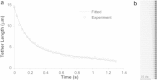
Membrane tethers are extracted when neutrophils roll on the endothelium to initiate their transendothelial migration. Tether extraction from both neutrophils and endothelial cells stabilizes neutrophil rolling, so it has been studied extensively and the force-velocity relationship for tether extraction is of great interest. Due to limitations of the techniques used in previous studies, this relationship has been obtained only from tethers perpendicular to the cell surface. Here, with the microcantilever technique, where latex beads affixed on silicon cantilevers were used as the force transducer, we extracted tethers either perpendicular or tangential to the neutrophil surface. We found that the force-velocity relationship was not sensitive to tether pulling direction. Little movement of the tether-cell junction was observed during tangential tether extraction, and no coalescence was observed during multiple tether extraction. Following adhesion rupture, spontaneous tether retraction was visualized by membrane staining, which revealed two phases: one was fast and exponential, whereas the other was slow and linear. Both phases can be reproduced with a mechanical model. These results show for the first time, to our knowledge, how neutrophil tethers shorten upon instantaneous force removal, and furthermore, they illustrate how membrane tethers contribute to neutrophil rolling stability during the inflammatory response.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Biomechanics of Neutrophil Tethers.Life (Basel). 2021 May 31;11(6):515. doi: 10.3390/life11060515. Life (Basel). 2021. PMID: 34073130 Free PMC article. Review.
-
Experimental studies of membrane tethers formed from human neutrophils.Ann Biomed Eng. 2002 Nov-Dec;30(10):1273-80. doi: 10.1114/1.1528614. Ann Biomed Eng. 2002. PMID: 12540203
-
Membrane tether extraction from human umbilical vein endothelial cells and its implication in leukocyte rolling.Biophys J. 2004 Nov;87(5):3561-8. doi: 10.1529/biophysj.104.047514. Epub 2004 Aug 31. Biophys J. 2004. PMID: 15339799 Free PMC article.
-
Simultaneous tether extraction contributes to neutrophil rolling stabilization: a model study.Biophys J. 2007 Jan 15;92(2):418-29. doi: 10.1529/biophysj.105.078808. Epub 2006 Oct 27. Biophys J. 2007. PMID: 17071668 Free PMC article.
-
Neutrophil rolling at high shear: flattening, catch bond behavior, tethers and slings.Mol Immunol. 2013 Aug;55(1):59-69. doi: 10.1016/j.molimm.2012.10.025. Epub 2012 Nov 9. Mol Immunol. 2013. PMID: 23141302 Free PMC article. Review.
Cited by
-
Membrane tension propagation couples axon growth and collateral branching.Sci Adv. 2022 Sep 2;8(35):eabo1297. doi: 10.1126/sciadv.abo1297. Epub 2022 Aug 31. Sci Adv. 2022. PMID: 36044581 Free PMC article.
-
Biomechanics of Neutrophil Tethers.Life (Basel). 2021 May 31;11(6):515. doi: 10.3390/life11060515. Life (Basel). 2021. PMID: 34073130 Free PMC article. Review.
-
The properties of chondrocyte membrane reservoirs and their role in impact-induced cell death.Biophys J. 2013 Oct 1;105(7):1590-600. doi: 10.1016/j.bpj.2013.08.035. Biophys J. 2013. PMID: 24094400 Free PMC article.
-
Microfluidics-based side view flow chamber reveals tether-to-sling transition in rolling neutrophils.Sci Rep. 2016 Jun 30;6:28870. doi: 10.1038/srep28870. Sci Rep. 2016. PMID: 27357741 Free PMC article.
-
Dynamics of membrane tethers reveal novel aspects of cytoskeleton-membrane interactions in axons.Biophys J. 2015 Feb 3;108(3):489-97. doi: 10.1016/j.bpj.2014.11.3480. Biophys J. 2015. PMID: 25650917 Free PMC article.
References
-
- Ley K., Laudanna C., Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. - PubMed
-
- Chen Y., Girdhar G., Shao J.Y. Single membrane tether extraction from adult and neonatal dermal microvascular endothelial cells. Am. J. Physiol. Cell Physiol. 2007;292:C1272–C1279. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

