Fibrin clot structure and mechanics associated with specific oxidation of methionine residues in fibrinogen
- PMID: 23283239
- PMCID: PMC3514520
- DOI: 10.1016/j.bpj.2012.10.036
Fibrin clot structure and mechanics associated with specific oxidation of methionine residues in fibrinogen
Abstract
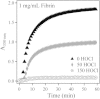
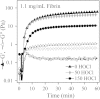
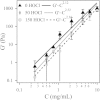
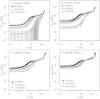
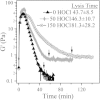
Using a combination of structural and mechanical characterization, we examine the effect of fibrinogen oxidation on the formation of fibrin clots. We find that treatment with hypochlorous acid preferentially oxidizes specific methionine residues on the α, β, and γ chains of fibrinogen. Oxidation is associated with the formation of a dense network of thin fibers after activation by thrombin. Additionally, both the linear and nonlinear mechanical properties of oxidized fibrin gels are found to be altered with oxidation. Finally, the structural modifications induced by oxidation are associated with delayed fibrin lysis via plasminogen and tissue plasminogen activator. Based on these results, we speculate that methionine oxidation of specific residues may be related to hindered lateral aggregation of protofibrils in fibrin gels.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Reduced plasminogen binding and delayed activation render γ'-fibrin more resistant to lysis than γA-fibrin.J Biol Chem. 2014 Oct 3;289(40):27494-503. doi: 10.1074/jbc.M114.588640. Epub 2014 Aug 15. J Biol Chem. 2014. PMID: 25128532 Free PMC article.
-
Fibrin self-assembly is adapted to oxidation.Free Radic Biol Med. 2016 Jun;95:55-64. doi: 10.1016/j.freeradbiomed.2016.03.005. Epub 2016 Mar 9. Free Radic Biol Med. 2016. PMID: 26969792
-
Tracking oxidation-induced alterations in fibrin clot formation by NMR-based methods.Sci Rep. 2021 Aug 3;11(1):15691. doi: 10.1038/s41598-021-94401-3. Sci Rep. 2021. PMID: 34344919 Free PMC article.
-
Fibrinogen and fibrin.Adv Protein Chem. 2005;70:247-99. doi: 10.1016/S0065-3233(05)70008-5. Adv Protein Chem. 2005. PMID: 15837518 Review.
-
Searching for differences between fibrinogen and fibrin that affect the initiation of fibrinolysis.Cardiovasc Hematol Agents Med Chem. 2008 Jul;6(3):181-9. doi: 10.2174/187152508784871954. Cardiovasc Hematol Agents Med Chem. 2008. PMID: 18673232 Review.
Cited by
-
Post-translational modifications of fibrinogen: implications for clotting, fibrin structure and degradation.Mol Biomed. 2024 Oct 31;5(1):45. doi: 10.1186/s43556-024-00214-x. Mol Biomed. 2024. PMID: 39477884 Free PMC article. Review.
-
Re-mining serum proteomics data reveals extensive post-translational modifications upon Zika and dengue infection.Mol Omics. 2023 May 9;19(4):308-320. doi: 10.1039/d2mo00258b. Mol Omics. 2023. PMID: 36810580 Free PMC article.
-
Lipopolysaccharide-binding protein (LBP) reverses the amyloid state of fibrin seen in plasma of type 2 diabetics with cardiovascular co-morbidities.Sci Rep. 2017 Aug 29;7(1):9680. doi: 10.1038/s41598-017-09860-4. Sci Rep. 2017. PMID: 28851981 Free PMC article.
-
Plasma-Derived Fibrin Hydrogels Containing Graphene Oxide for Infections Treatment.ACS Mater Lett. 2023 Mar 23;5(4):1245-1255. doi: 10.1021/acsmaterialslett.2c01044. eCollection 2023 Apr 3. ACS Mater Lett. 2023. PMID: 38323142 Free PMC article.
-
Computational Simulations of Thrombolytic Therapy in Acute Ischaemic Stroke.Sci Rep. 2018 Oct 25;8(1):15810. doi: 10.1038/s41598-018-34082-7. Sci Rep. 2018. PMID: 30361673 Free PMC article.
References
-
- Doolittle R.F. Fibrinogen and fibrin. Annu. Rev. Biochem. 1984;53:195–229. - PubMed
-
- Pretorius E., Steyn H., Oberholzer H.M. Differences in fibrin fiber diameters in healthy individuals and thromboembolic ischemic stroke patients. Blood Coagul. Fibrinolysis. 2011;22:696–700. - PubMed
-
- Colle J.P., Mishal Z., Soria C. Abnormal fibrin clot architecture in nephrotic patients is related to hypofibrinolysis: influence of plasma biochemical modifications: a possible mechanism for the high thrombotic tendency? Thromb. Haemost. 1999;82:1482–1489. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

