A novel AMPK-dependent FoxO3A-SIRT3 intramitochondrial complex sensing glucose levels
- PMID: 23283301
- PMCID: PMC11113715
- DOI: 10.1007/s00018-012-1244-6
A novel AMPK-dependent FoxO3A-SIRT3 intramitochondrial complex sensing glucose levels
Abstract
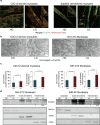
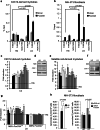
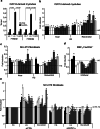
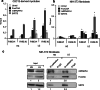
Reduction of nutrient intake without malnutrition positively influences lifespan and healthspan from yeast to mice and exerts some beneficial effects also in humans. The AMPK-FoxO axis is one of the evolutionarily conserved nutrient-sensing pathways, and the FOXO3A locus is associated with human longevity. Interestingly, FoxO3A has been reported to be also a mitochondrial protein in mammalian cells and tissues. Here we report that glucose restriction triggers FoxO3A accumulation into mitochondria of fibroblasts and skeletal myotubes in an AMPK-dependent manner. A low-glucose regimen induces the formation of a protein complex containing FoxO3A, SIRT3, and mitochondrial RNA polymerase (mtRNAPol) at mitochondrial DNA-regulatory regions causing activation of the mitochondrial genome and a subsequent increase in mitochondrial respiration. Consistently, mitochondrial transcription increases in skeletal muscle of fasted mice, with a mitochondrial DNA-bound FoxO3A/SIRT3/mtRNAPol complex detectable also in vivo. Our results unveil a mitochondrial arm of the AMPK-FoxO3A axis acting as a recovery mechanism to sustain energy metabolism upon nutrient restriction.
Figures



References
-
- McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. J Nutr. 1935;10:63–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

